The primary disadvantages of a crucible are not inherent to the tool itself, but to the physical and chemical limitations of the materials used to construct it. No single crucible material is perfect for every application, leading to critical trade-offs regarding thermal shock, chemical reactivity, lifespan, and cost that can result in process failure or product contamination if ignored.
The core issue is that the "ideal" crucible—completely inert, infinitely durable, and impervious to extreme temperature change—does not exist. Every real-world crucible is a compromise, and its disadvantages are the specific ways in which it falls short of this ideal for a given task.

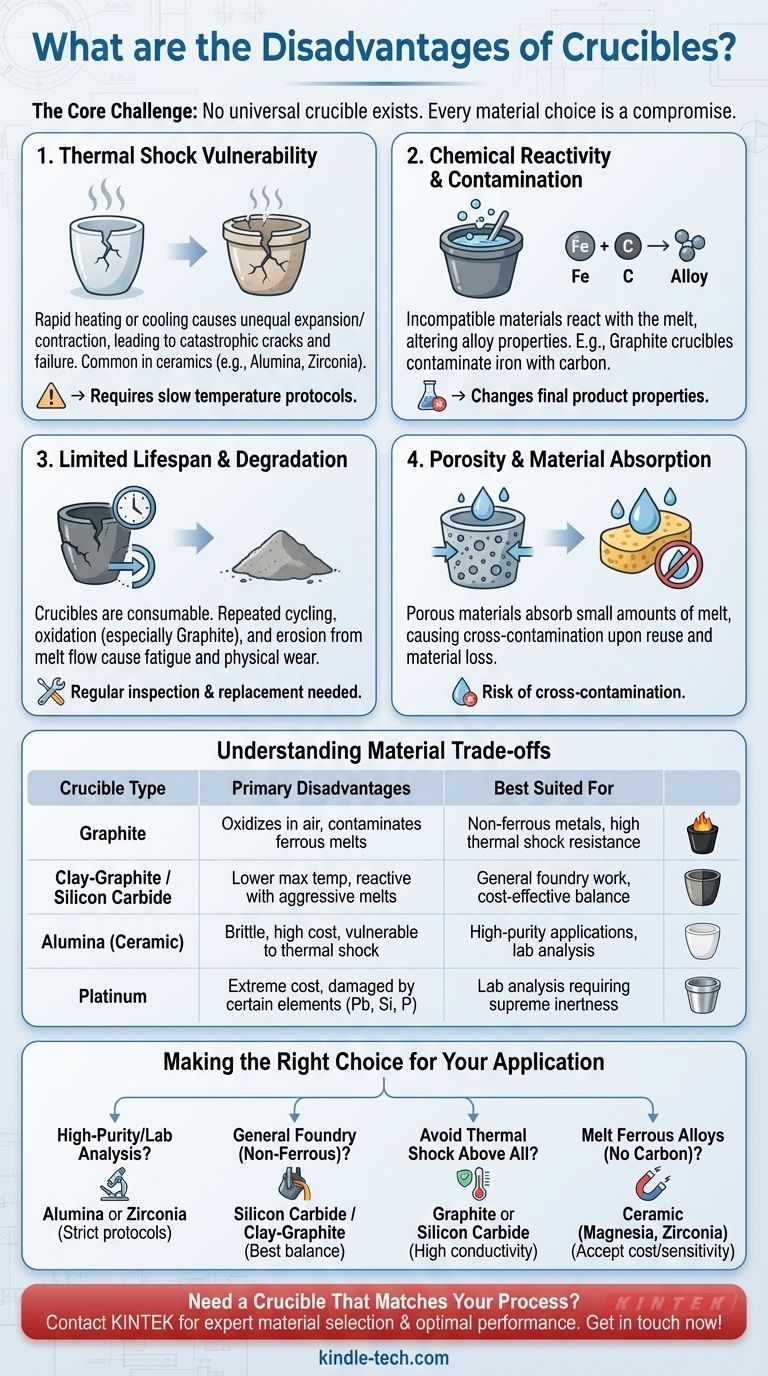
The Core Challenge: No Universal Crucible Exists
The perfect crucible would possess immense temperature resistance, be chemically inert to any substance, and withstand rapid heating and cooling indefinitely. In practice, every material choice represents a set of compromises.
Thermal Shock Vulnerability
A primary disadvantage of many ceramic crucibles (like alumina or zirconia) is their susceptibility to thermal shock.
This occurs when the crucible is heated or cooled too rapidly, causing different parts of the material to expand or contract at different rates. The resulting internal stress can cause catastrophic failure, leading to cracks and a complete loss of the molten contents.
Chemical Reactivity and Contamination
A crucible that is not perfectly compatible with its contents will react with the melt. This is a critical failure mode.
For example, using a graphite crucible to melt iron will cause carbon to dissolve into the melt, changing the final alloy's properties. Similarly, aggressive fluxes or certain metals can actively corrode or "wet" the crucible walls, degrading the crucible and contaminating the product.
Limited Lifespan and Degradation
Crucibles are consumable items with a finite lifespan. They are not permanent tools.
Repeated thermal cycling, even when done carefully, causes micro-fractures and fatigue. Exposure to air at high temperatures can cause oxidation (especially in graphite crucibles), while the flow of molten material can cause physical erosion. This degradation requires regular inspection and replacement, adding to operational costs.
Porosity and Material Absorption
Some crucible materials, particularly certain grades of ceramics, can be slightly porous.
This porosity allows a small amount of the molten material to be absorbed into the crucible walls. This can lead to cross-contamination if the crucible is reused for a different alloy or compound. It also represents a loss of valuable material.
Understanding the Material Trade-offs
The disadvantages become clear when comparing common crucible types. The right choice for one process is often the wrong choice for another.
Graphite Crucibles
These offer excellent thermal conductivity, which makes them highly resistant to thermal shock. However, they readily oxidize in air at high temperatures and will contaminate any melt that readily absorbs carbon, such as ferrous metals.
Clay-Graphite and Silicon Carbide
These are the workhorses of many foundries. They offer a good balance of thermal shock resistance, durability, and cost. Their main disadvantage is a lower maximum operating temperature compared to pure ceramics and potential reactivity with highly aggressive melts.
Alumina (Ceramic) Crucibles
Excellent for high-purity applications due to their chemical inertness and very high melting point. Their key disadvantages are high cost, extreme brittleness, and a significant vulnerability to thermal shock if not handled with precise temperature control.
Platinum Crucibles
For laboratory analysis, platinum offers supreme chemical inertness and a very high melting point. Its overwhelming disadvantages are its prohibitive cost and susceptibility to damage from certain elements (like lead, silicon, and phosphorus) at high temperatures.
Making the Right Choice for Your Application
Selecting a crucible requires matching its known limitations to the demands of your specific process.
- If your primary focus is high-purity melts or lab analysis: Choose high-purity alumina or zirconia, but implement strict, slow heating and cooling protocols to prevent thermal shock.
- If your primary focus is general foundry work for non-ferrous metals: A silicon carbide or clay-graphite crucible provides the best balance of cost, durability, and performance.
- If your primary focus is avoiding thermal shock above all else: A graphite or silicon carbide crucible is the superior choice due to its high thermal conductivity.
- If your primary focus is melting ferrous alloys without carbon contamination: You must use a ceramic crucible, such as one made from magnesia or zirconia, and accept its higher cost and thermal sensitivity.
Understanding a crucible's disadvantages is the first step toward ensuring a safe, successful, and contamination-free high-temperature process.
Summary Table:
| Crucible Type | Primary Disadvantages | Best Suited For |
|---|---|---|
| Graphite | Oxidizes in air, contaminates ferrous melts | Non-ferrous metals, thermal shock resistance |
| Clay-Graphite/Silicon Carbide | Lower max temperature, reactivity with aggressive melts | General foundry work, cost-effective balance |
| Alumina (Ceramic) | Brittle, high cost, vulnerable to thermal shock | High-purity applications, lab analysis |
| Platinum | Extremely high cost, damaged by certain elements | Lab analysis requiring supreme inertness |
Need a Crucible That Matches Your Specific Process?
Choosing the wrong crucible can lead to contamination, equipment failure, and costly downtime. At KINTEK, we specialize in providing laboratory equipment and consumables tailored to your unique high-temperature applications. Our experts will help you select the ideal crucible material—whether you require high purity, thermal shock resistance, or chemical inertness—ensuring optimal performance and reliability for your lab or foundry.
Contact us today to discuss your needs and let KINTEK enhance your process efficiency and safety. Get in touch now!
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- What are the advantages of using graphite crucibles in 3000°C experiments? Achieve Superior Purity and Performance
- What is the most durable crucible? Match the Right Crucible to Your Melting Application
- What are the functions of alumina crucibles in LLZO sintering? Ensure Li-Rich Atmosphere for Stable Cubic Phases
- What are high temperature crucibles made of? Choose the Right Material for Your Lab
- Why are alumina crucibles recommended over quartz crucibles for liquid aluminum? Ensure Experimental Accuracy
- What is the process of a crucible furnace? A Step-by-Step Guide to Small-Batch Melting
- Why are graphite crucibles selected as melting vessels for AlMgZn cross-over alloys? Essential Benefits & Purity Tips
- What are the different sizes of crucibles? A Guide from Jewelry to Industrial Scales



















