In short, the porosity of ceramic ware is determined by three primary factors: the composition of the clay body, the peak temperature it is fired to, and the amount of time it is held at that temperature. These elements collectively control the degree of vitrification—the process where clay particles melt, fuse, and form glass, which in turn seals the pores within the ceramic body.
The core challenge in controlling ceramic porosity is not just about choosing the right materials, but about precisely managing the firing process. You are essentially controlling how much of the clay body transforms into glass, with the goal of balancing porosity against other critical properties like strength and thermal shock resistance.

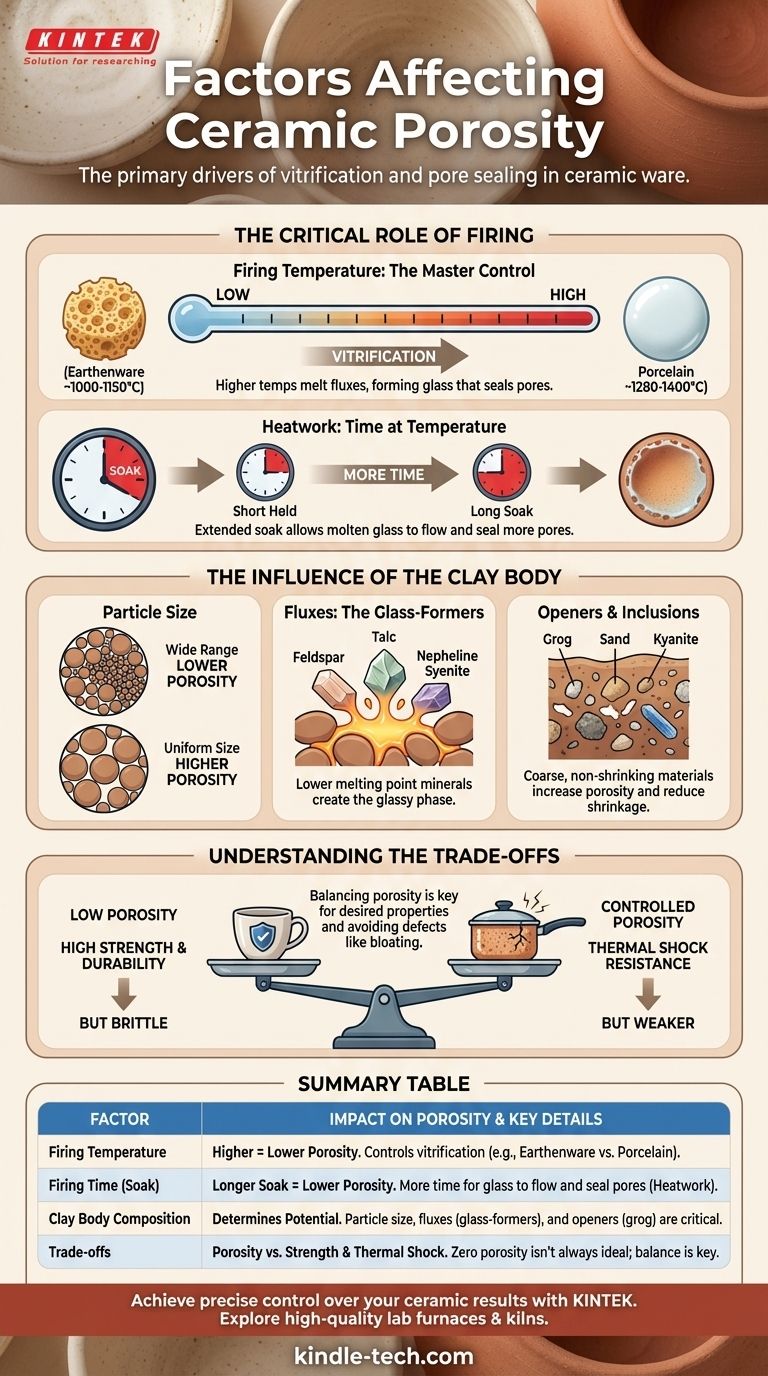
The Critical Role of Firing
The firing cycle is the most significant factor you can control to influence the final porosity of a piece. It's not just about how hot the kiln gets, but how the heat is applied over time.
Firing Temperature: The Master Control
The peak temperature reached during firing has the most direct impact on porosity. As temperatures rise, specific minerals in the clay body, called fluxes, begin to melt and form liquid glass.
This glass flows into the voids between the more refractory (heat-resistant) particles, pulling them closer together and sealing the pores. Higher temperatures create more liquid glass, resulting in a denser, less porous body.
- Earthenware: Fired at low temperatures (approx. 1000-1150°C), it undergoes minimal vitrification and remains highly porous (10-15% water absorption).
- Stoneware: Fired at higher temperatures (approx. 1200-1300°C), it becomes partially or fully vitrified, making it very strong and minimally porous (0.5-2% water absorption).
- Porcelain: Fired to the highest temperatures (approx. 1280-1400°C), it becomes fully vitrified and translucent, with near-zero porosity (<0.5% absorption).
Heatwork: It's Time at Temperature
Heatwork is the combined effect of temperature and time. A ceramic piece held at its peak temperature for an extended period (a "soak" or "hold") will become more vitrified than a piece brought to the same temperature and immediately cooled.
This soaking period gives the molten glass more time to flow, mature, and seal the remaining pores, effectively reducing porosity.
The Influence of the Clay Body
The recipe of the clay itself predetermines its potential for vitrification. Different ingredients play specific roles in either promoting or inhibiting the sealing of pores.
Particle Size
A clay body with a wide range of particle sizes will pack together more densely in its unfired (greenware) state. The smaller particles fill the gaps between larger ones, leaving less empty space to be filled during firing and resulting in lower final porosity.
Fluxes: The Glass-Formers
Fluxes are minerals like feldspar, nepheline syenite, or talc that have a lower melting point than clay. They are the first ingredients to melt in the kiln, creating the glassy phase that binds everything else together.
Increasing the amount of flux in a clay body will allow it to become dense and non-porous at a lower temperature.
Openers and Inclusions
Materials like grog (pre-fired and ground clay), sand, or kyanite are added to a clay body to increase its porosity and reduce shrinkage. These coarse, non-shrinking particles create a more open structure and resist the vitrification process.
This controlled porosity can be highly desirable, as it improves the clay's drying properties and its ability to withstand thermal shock.
Understanding the Trade-offs
Manipulating porosity is always a balancing act. Reducing porosity to zero is not always the ideal outcome, as it can negatively affect other desired properties.
Porosity vs. Strength
Generally, as porosity decreases, the mechanical strength and durability of the ceramic ware increase. The glassy bond in a fully vitrified body like porcelain makes it incredibly strong and resistant to chipping. Porous earthenware, by contrast, is much weaker.
Porosity vs. Thermal Shock Resistance
A completely dense, vitrified body can be brittle and prone to cracking when subjected to rapid temperature changes. The small, empty voids in a slightly more porous body (often one containing grog) can act as crack-arrestors, stopping a micro-fracture from propagating through the piece. This is why cookware and raku bodies are intentionally designed with some porosity.
The Danger of Over-firing
Pushing a clay body past its ideal maturation point in pursuit of zero porosity can lead to bloating. Trapped gases within the melting body expand, creating large bubbles and voids. This ironically increases the overall porosity and severely weakens the structure, often ruining the piece.
Making the Right Choice for Your Goal
Ultimately, the ideal porosity is determined by the intended function of the ceramic ware.
- If your primary focus is food safety and durability (dinnerware, mugs): Aim for full vitrification by using a stoneware or porcelain body and firing it to its proper maturation temperature.
- If your primary focus is thermal shock resistance (cookware, pizza stones): Use a specialized clay body containing grog or other tempers to maintain a controlled level of porosity.
- If your primary focus is decorative or horticultural (sculptures, planters): A low-fire earthenware body is perfectly suitable, as its high porosity is not a functional drawback and can even be beneficial for plant roots.
- If you need a waterproof surface on a porous body: Rely on a well-fitted glaze that forms an impermeable glassy layer over the earthenware, but be aware that any cracks or chips in the glaze will expose the absorbent clay underneath.
By understanding these interconnected factors, you can move from simply following a recipe to making intentional choices that produce strong, beautiful, and functional ceramic ware.
Summary Table:
| Factor | Impact on Porosity | Key Details |
|---|---|---|
| Firing Temperature | Higher temperature = lower porosity | Controls vitrification; e.g., Earthenware (porous) vs. Porcelain (dense). |
| Firing Time (Soak) | Longer soak = lower porosity | More time for glass to flow and seal pores (heatwork). |
| Clay Body Composition | Determines potential porosity | Particle size, fluxes (e.g., feldspar), and openers (e.g., grog) are critical. |
| Trade-offs | Porosity vs. Strength & Thermal Shock | Zero porosity isn't always ideal; balance is key for functionality. |
Achieve precise control over your ceramic results with KINTEK. Whether you're a studio artist, a production potter, or a research lab, the right equipment is essential for mastering firing cycles and material behavior. KINTEK specializes in high-quality lab furnaces, kilns, and consumables designed for reliability and precision. Let our expertise help you perfect your process—contact our specialists today to discuss your specific needs and find the ideal solution for your ceramic work.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Is silicon carbide corrosion-resistant? Unlock Its Power in Extreme Chemical & Thermal Environments
- Why is ceramic sintering used? To transform fragile powder into a strong, dense solid.
- What are the methods of high temperature ceramic? Master the 3-Stage Process for Durable Components
- What is the purpose of firing or sintering? To Transform Weak Powder into Strong, Dense Ceramics
- What is the ceramic tube high temperature? From 1100°C to 1800°C, Choose the Right Material
- How much temperature can porcelain withstand? Unlock Its True Heat Resistance & Avoid Thermal Shock
- What role does polyurethane foam play as a sacrificial template? Create Advanced Porous MgO Ceramics
- What are the three types of dental ceramic? A Guide to Balancing Aesthetics & Strength



















