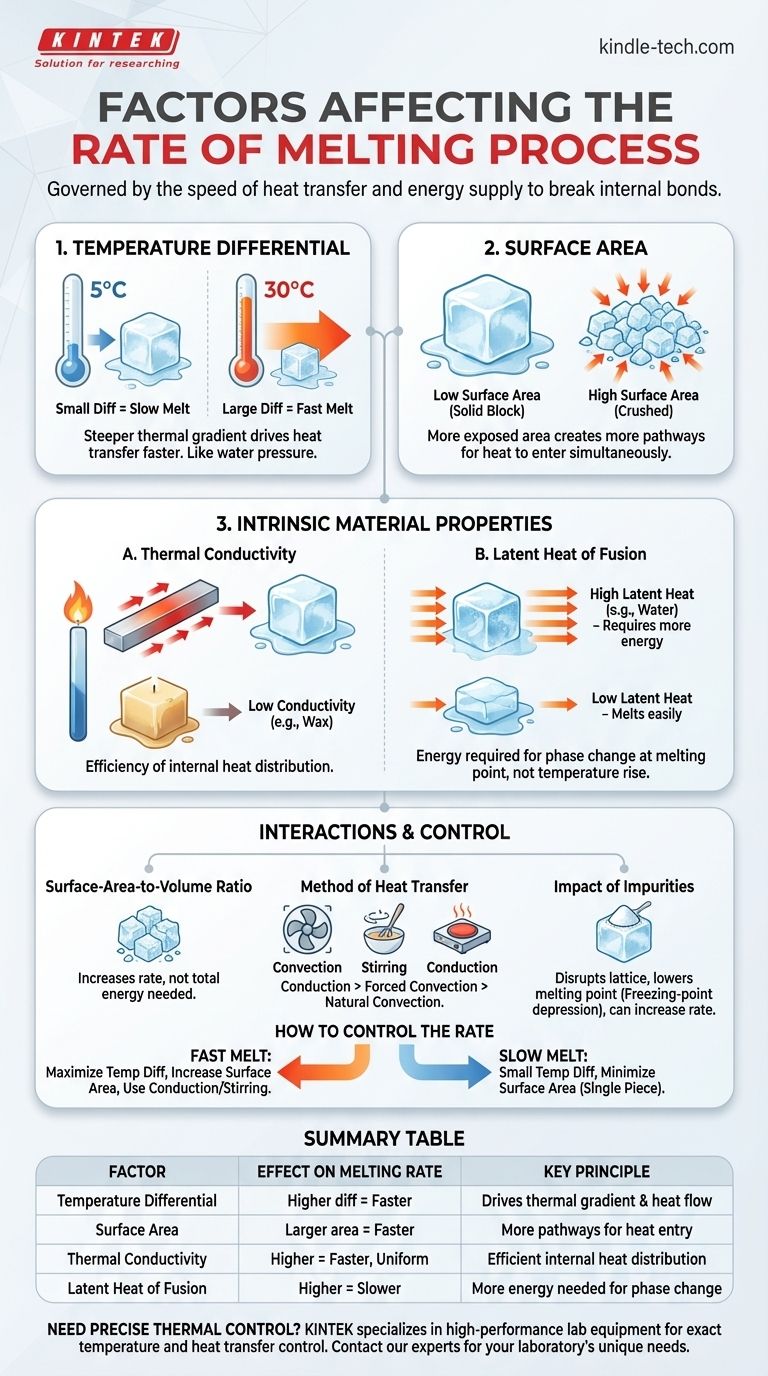
The rate at which a solid melts is governed by the speed of heat transfer into the substance. This process is primarily dictated by the temperature difference between the object and its environment, its exposed surface area, its total volume, and intrinsic material properties like thermal conductivity and latent heat of fusion.
The core principle is simple: melting is a battle against a substance's internal bonds, and the speed of that process depends entirely on how quickly you can supply the energy needed to break them. To melt something faster, you must increase the rate at which heat energy flows from the surroundings into the material.

The Physics of Heat Transfer in Melting
Melting is a phase transition from a solid to a liquid. This change requires energy to overcome the molecular forces that hold the solid in a fixed, crystalline structure. The speed of melting is therefore not a property of the material alone, but a function of how fast this required energy can be absorbed.
Temperature Differential
The single most significant factor is the temperature difference between the substance and its surroundings. A larger differential creates a steeper "thermal gradient," driving heat to transfer more rapidly.
Think of it like water pressure. A small height difference results in a slow trickle, while a large height difference creates a powerful flow. Similarly, an ice cube in a 30°C room will melt much faster than one in a 5°C room.
Surface Area
Heat is absorbed through the surface of an object. By increasing the surface area exposed to the warmer environment, you create more pathways for heat to enter simultaneously.
This is why crushed ice cools a drink much faster than a single large ice cube of the same total mass. The crushed ice has a vastly larger surface-area-to-volume ratio, allowing for a much higher rate of heat absorption.
Intrinsic Material Properties
Two key properties of the material itself dictate how it responds to heat.
Thermal Conductivity
Thermal conductivity is a measure of how efficiently a material transfers heat from its surface to its interior. Materials with high thermal conductivity, like metals, distribute absorbed heat quickly throughout their entire volume, leading to a faster and more uniform melt.
Materials with low conductivity, like plastics or wax, are thermal insulators. Heat penetrates them slowly, so they tend to melt layer by layer from the outside in.
Latent Heat of Fusion
Latent heat of fusion is the amount of "hidden" energy required to change a substance from solid to liquid at its melting point. During the phase change, all absorbed energy is used to break molecular bonds, not to raise the temperature.
A substance with a high latent heat of fusion requires a large amount of energy to melt. Water, for instance, has a very high latent heat, which is why ice is so effective at cooling things—it absorbs a great deal of heat before it fully melts.
Understanding the Trade-offs
These factors do not operate in isolation. Their interaction determines the final outcome, and understanding these relationships is key to controlling the process.
The Surface-Area-to-Volume Ratio
While increasing surface area (by crushing or shredding) dramatically increases the melting rate, it does not change the total volume or the total amount of energy needed (the latent heat). You are simply opening more "doors" for that energy to enter at once.
The Method of Heat Transfer
How the heat is delivered matters. An object melting in still air relies on natural convection and radiation, which is relatively slow. Stirring a liquid around a melting solid introduces forced convection, dramatically accelerating heat transfer and the melting rate. Direct conduction, like placing an ice cube on a warm metal plate, is often the fastest method.
The Impact of Impurities
Impurities in a substance can disrupt its crystal lattice, typically lowering its melting point. This is known as freezing-point depression. By lowering the temperature at which melting begins, impurities can effectively increase the temperature differential between the substance and its environment, thus increasing the melting rate (e.g., salting an icy road).
How to Control the Rate of Melting
Your strategy should align directly with your goal for the melting process.
- If your primary focus is to melt a substance as fast as possible: Maximize the temperature difference, break the substance into the smallest possible pieces to increase surface area, and use a method of heating that involves forced convection (like stirring) or direct conduction.
- If your primary focus is a slow, controlled melt (e.g., tempering chocolate): Use a small and stable temperature difference (like a double boiler) and keep the substance in a larger, single piece to minimize the surface-area-to-volume ratio.
- If your primary focus is selecting a material for a specific application: For tasks requiring rapid melting, choose materials with low latent heat of fusion and high thermal conductivity. For applications requiring resistance to melting, choose materials with the opposite properties.
By understanding these core principles of heat transfer, you gain the ability to precisely control any melting process to achieve your desired outcome.
Summary Table:
| Factor | Effect on Melting Rate | Key Principle |
|---|---|---|
| Temperature Differential | Higher difference = Faster melting | Drives the thermal gradient and heat flow |
| Surface Area | Larger area = Faster melting | More pathways for heat to enter the material |
| Thermal Conductivity | Higher conductivity = Faster, more uniform melting | Efficient internal heat distribution |
| Latent Heat of Fusion | Higher latent heat = Slower melting | More energy required for the phase change |
Need precise thermal control for your lab processes? The principles of heat transfer are fundamental to efficient melting, mixing, and synthesis. At KINTEK, we specialize in high-performance lab equipment, including heating mantles, hot plates, and furnaces, designed to give you exact control over temperature and heat transfer. Whether you're developing new materials or running critical assays, our solutions help you achieve faster, more consistent, and safer results. Contact our experts today to find the perfect heating solution for your laboratory's unique needs.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is sintering in induction furnace? Mastering the Thermal Process for Durable Materials
- What is the function of asbestos boards in the induction furnace lining? Essential Insulation & Moisture Control
- What does an induction furnace make use of? Harnessing Electromagnetic Induction for Clean, Efficient Melting
- What is the working principle of induction furnace? Achieve Fast, Efficient Metal Melting
- How thick is the lining of an induction furnace? Optimize Safety, Efficiency, and Lifespan
- What is the cost of an induction furnace? A Guide to Budgeting for Your Melting System
- What key environmental conditions does a vacuum induction furnace provide for the synthesis of titanium oxycarbide?
- How many times can metal be melted down and used again? The Key to Infinite Recyclability



















