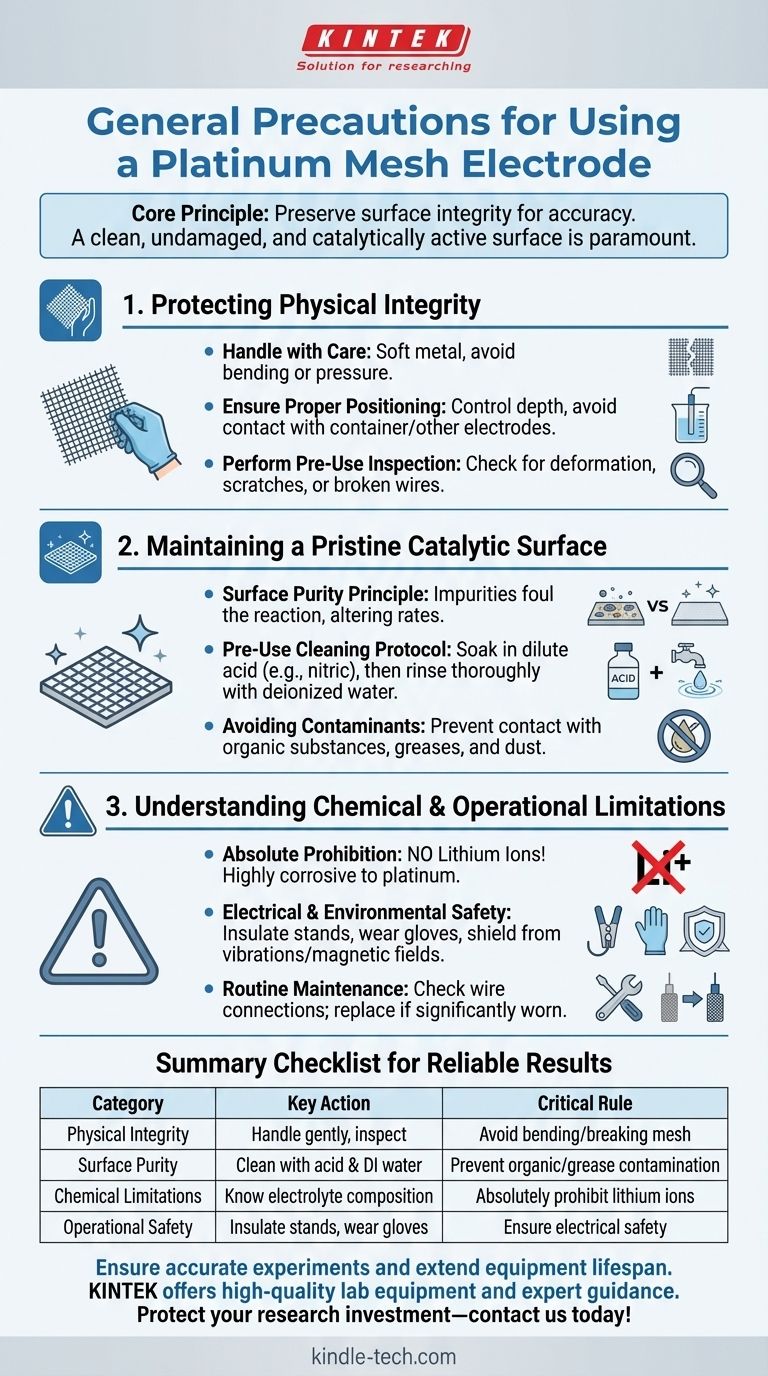
To properly use a platinum mesh electrode, you must focus on three primary areas of precaution. Handle it with extreme care to prevent mechanical damage, maintain a pristine surface by avoiding chemical contamination, and strictly prohibit its use with lithium ions, which are corrosive to platinum.
The core principle behind all platinum electrode precautions is the preservation of its surface integrity. An electrode's value and accuracy depend entirely on a clean, undamaged, and catalytically active surface, making physical and chemical purity your highest priorities.

Protecting the Electrode's Physical Integrity
A platinum mesh electrode is a delicate and expensive instrument. Its physical structure is directly linked to its performance and lifespan.
Handle with Care
Platinum is a soft metal. The mesh is particularly susceptible to being bent, warped, or having its wires broken.
Always handle the electrode gently. Avoid dropping, impacting, or applying any significant pressure to the mesh structure.
Ensure Proper Positioning
Incorrect placement within an electrochemical cell can lead to damage or inaccurate readings.
When inserting the electrode into the electrolyte, carefully control the depth. Ensure it does not touch the bottom of the container or make contact with other electrodes, which could cause a short circuit or interfere with measurements.
Perform a Pre-Use Inspection
Never assume an electrode is ready for use, especially if it is shared equipment.
Before each experiment, visually inspect the mesh for any signs of deformation, scratches, or broken wires. A damaged electrode will not provide reliable or repeatable results.
Maintaining a Pristine Catalytic Surface
The entire function of an electrode relies on the chemical reactions occurring at its surface. Contamination, even at a microscopic level, can render your results invalid.
The Principle of Surface Purity
Think of the platinum surface as a perfectly clean workbench for your chemical reaction. Any impurity, from organic residue to dust, occupies space and prevents the desired reaction from occurring correctly.
This "fouling" can alter reaction rates, shift potentials, and introduce significant errors into your measurements.
Pre-Use Cleaning Protocol
To ensure a clean, active surface, a standard cleaning procedure is essential before every use.
Soak the electrode in a dilute acid, such as dilute nitric acid, to remove surface oxides and inorganic impurities. Following the acid wash, rinse it thoroughly with deionized water.
Avoiding Contaminants During Use
Prevention is the most effective maintenance strategy. Be vigilant about what comes into contact with the electrode.
Avoid all contact with organic substances, greases, or any other material that is not a planned part of your experiment.
Understanding Chemical and Operational Limitations
Using the electrode outside of its intended chemical or physical environment can cause irreversible damage or compromise your data.
The Absolute Prohibition: Lithium Ions
This is a critical, non-negotiable rule for working with platinum.
Do not allow the electrode to come into contact with lithium ions. Lithium is highly corrosive to platinum and will permanently damage the electrode.
Electrical and Environmental Safety
Proper setup is critical for both personal safety and data integrity.
Ensure any metal stands or clamps are properly insulated to prevent electric shock. Always wear insulating gloves and never handle the apparatus with wet hands. For the most sensitive measurements, shield the setup from mechanical vibrations and external magnetic fields to maintain a stable environment.
Routine Maintenance and Inspection
Long-term reliability depends on consistent care.
Periodically check all wire connections to ensure good electrical conductivity. If, after cleaning, the electrode's performance is still degraded or it shows significant physical wear, it must be replaced.
A Checklist for Reliable Results
Your specific priorities will determine which precautions require the most attention. Use this guide to align your actions with your goals.
- If your primary focus is maximum accuracy and repeatability: Your highest priority is the rigorous pre-use cleaning protocol and shielding the experiment from environmental disturbances.
- If your primary focus is extending the electrode's lifespan: Concentrate on gentle physical handling, meticulous positioning, and strict avoidance of prohibited chemicals like lithium ions.
- If your primary focus is operational safety: The non-negotiable steps are ensuring proper electrical insulation of all components and always wearing protective gloves.
Ultimately, treating your platinum mesh electrode with methodical care is the only way to protect your investment and ensure the integrity of your scientific work.
Summary Table:
| Precaution Category | Key Action | Critical Rule to Follow |
|---|---|---|
| Physical Integrity | Handle gently, inspect before use | Avoid bending, warping, or breaking the delicate mesh |
| Surface Purity | Clean with dilute acid & deionized water before each use | Prevent contamination from organics, grease, and dust |
| Chemical Limitations | Know your electrolyte composition | Absolutely prohibit contact with lithium ions (corrosive) |
| Operational Safety | Insulate stands, wear gloves, shield from vibrations | Ensure electrical safety and stable measurement environment |
Ensure your lab's electrochemical experiments are accurate and your equipment lasts longer. Proper care of sensitive electrodes is crucial for reliable data. KINTEK specializes in high-quality lab equipment and consumables, including electrodes designed for durability and performance. Our experts can help you select the right tools and provide guidance on best practices for maintenance. Protect your research investment—contact our team today for a consultation!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance



















