For successful brazing, the key heat requirement is not a single temperature but a complete thermal profile. You must heat the assembly to a temperature slightly above the filler metal’s melting point but below the base materials' melting point, and hold it for a specific duration (dwell time). This process must occur in a controlled atmosphere to allow the filler metal to flow properly and form a strong joint.
Achieving a high-quality braze depends less on hitting one specific temperature and more on precisely managing the relationship between temperature, time, and atmosphere throughout the entire heating and cooling cycle.
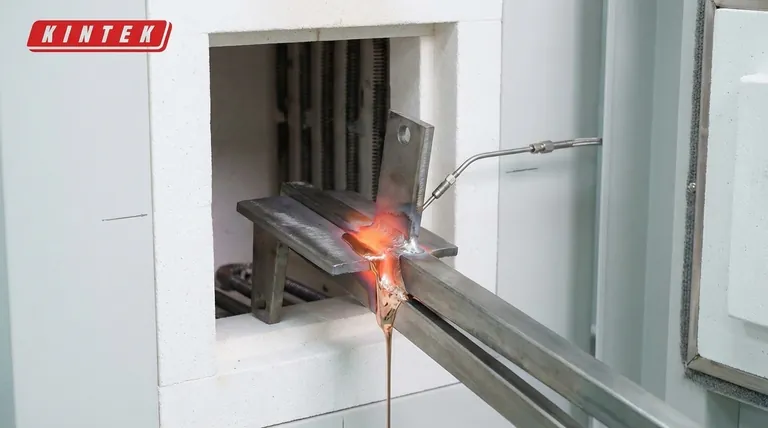
The Core Elements of the Brazing Cycle
The application of heat in brazing is a dynamic process known as the brazing cycle or thermal profile. It consists of more than just a peak temperature.
Reaching the Brazing Temperature
The primary goal is to heat the entire assembly uniformly to the specified brazing temperature. This temperature is chosen to be above the liquidus (the point at which the filler metal becomes completely liquid).
This ensures the filler alloy can flow freely via capillary action into the tight gap between the base materials.
The Critical Role of Dwell Time
Simply reaching the temperature is not enough. The assembly must be held at that temperature for a specific period, known as the dwell time.
This balance between time and temperature is critical. A sufficient dwell time allows the liquid filler metal to fully penetrate the joint, creating a void-free bond. Optimizing this, for example, by using longer dwell times, can significantly reduce scrap and improve product consistency.
Managing Heating and Cooling Rates
How quickly you heat the parts (ramp rate) and how quickly you cool them is also part of the heat requirement. Rapid or uneven heating can cause distortion, while improper cooling can introduce residual stresses that weaken the final assembly.
Why Atmosphere is as Important as Heat
Applying heat in an uncontrolled environment will result in a failed braze. Heat accelerates chemical reactions, and the most significant one to control is oxidation.
Preventing Oxidation
As the base metals heat up, they will readily form oxides on their surface if exposed to air. This oxide layer acts as a barrier, preventing the liquid filler metal from "wetting" or bonding to the base materials.
A successful braze requires the surfaces to be perfectly clean on a microscopic level, a condition that is impossible to maintain with heat unless the atmosphere is controlled.
Key Atmospheric Conditions
To prevent oxidation, brazing is typically performed in a controlled atmosphere furnace. The environment must be clean and dry.
Ideal conditions often include a dew point of -40°C or lower (indicating very low water vapor) and an oxygen level below 100 parts per million (ppm).
The Function of Inert Gas
This protective environment is usually created by purging the furnace with an inert gas, most commonly nitrogen. This gas displaces the oxygen and moisture, ensuring the heat can perform its function without causing destructive oxidation.
Understanding the Trade-offs
Optimizing the brazing cycle requires balancing competing factors. Deviating from the ideal parameters introduces significant risks.
Too Much Heat or Time
Excessive temperature or an overly long dwell time can be destructive. It can cause the base metal's grain structure to grow, reducing its strength. In some cases, it can even lead to erosion, where the liquid filler metal begins to dissolve the base material.
Not Enough Heat or Time
Insufficient heat or dwell time is a more common cause of failure. If the filler metal doesn't become fully liquid or doesn't have time to flow, the joint will be incomplete. This results in voids, low strength, and potential leak paths.
Inadequate Atmospheric Control
If the atmospheric controls fail—for instance, if the dew point is too high or oxygen leaks into the furnace—the braze will fail regardless of the time and temperature settings. The parts will oxidize, and the filler metal will not flow into the joint.
Making the Right Choice for Your Goal
Achieving a perfect braze requires a holistic view of the process. Your specific priority will determine where you focus your attention.
- If your primary focus is joint strength and integrity: Concentrate on achieving the correct dwell time at a temperature that ensures the filler metal is fully liquid, allowing for complete capillary flow.
- If your primary focus is high-volume production and low scrap: Invest in precise process control to maintain a consistent time-temperature profile and a pure furnace atmosphere for every cycle.
- If your primary focus is preventing part failure: Ensure your furnace atmosphere is validated to be clean and dry (low dew point, low O2) before you even begin to optimize the thermal profile.
Ultimately, mastering brazing requires treating heat, time, and atmosphere as an interconnected system, not as separate variables.
Summary Table:
| Brazing Parameter | Key Requirement | Purpose |
|---|---|---|
| Brazing Temperature | Above filler metal liquidus, below base metal melting point | Ensures filler metal flows via capillary action |
| Dwell Time | Specific duration at brazing temperature | Allows for complete joint penetration and bonding |
| Atmosphere | Dew point ≤ -40°C, O₂ ≤ 100 ppm | Prevents surface oxidation for proper wetting |
| Heating/Cooling Rate | Controlled and uniform | Prevents part distortion and residual stresses |
Achieve flawless, high-strength brazed joints with KINTEK.
Precisely controlling the brazing cycle—temperature, time, and atmosphere—is critical for your product's integrity and production yield. KINTEK specializes in advanced laboratory furnaces and atmosphere control systems designed specifically for reliable, repeatable brazing processes.
Our solutions help you:
- Eliminate joint failures and scrap by maintaining optimal atmospheric purity (low dew point, low O₂).
- Ensure consistent results batch after batch with precise thermal profiling.
- Protect your base materials from issues like erosion and grain growth.
Whether your priority is ultimate joint strength or high-volume production efficiency, KINTEK has the expertise and equipment to meet your laboratory's brazing needs.
Contact KINTEK today to discuss how our lab equipment can optimize your brazing process.
Visual Guide
Related Products
- 1200℃ Muffle Furnace Oven for Laboratory
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
- Large Vertical Graphite Vacuum Graphitization Furnace
- Vacuum Heat Treat and Pressure Sintering Furnace for High Temperature Applications
People Also Ask
- What is the process of bio-oil production? A Step-by-Step Guide to Pyrolysis
- What are the benefits of metallurgy? Achieve Superior Material Performance and Efficiency
- Is powder metallurgy the same as sintering? The Critical Step That Bonds Metal Powder into Strong Parts
- What are the advantages of physical vapour deposition method? Achieve Superior, Durable Surface Coatings
- What is DFT coating thickness? Ensure Quality and Performance with Precise Measurement
- What is sputtering yield? Master the Key to Efficient Thin Film Deposition
- Can pyrolysis produce electricity? Unlock the Potential of Waste-to-Energy Systems
- What materials are direct energy deposition? Key Metals & Alloys for High-Performance 3D Printing


















