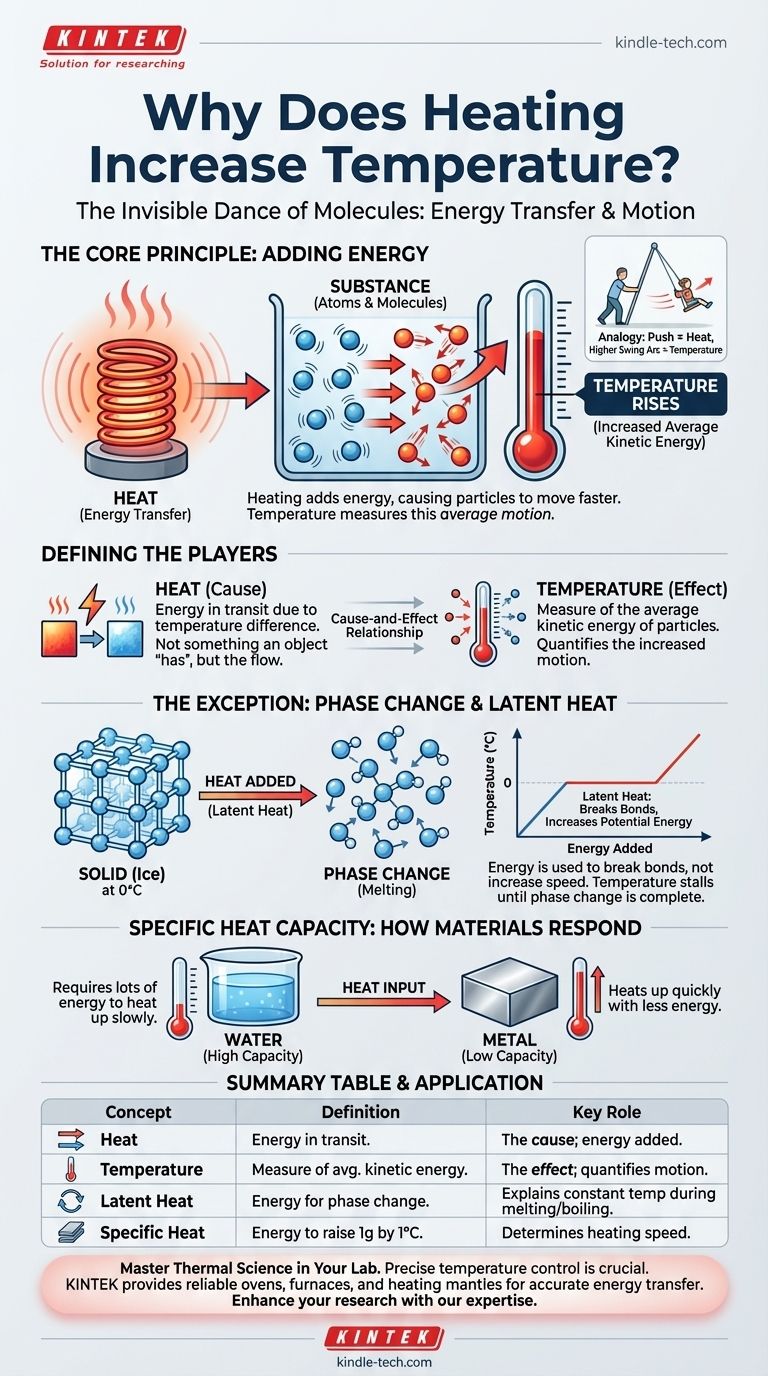
At its core, heating increases temperature because you are adding energy to a substance, causing its fundamental particles—atoms and molecules—to move, vibrate, or rotate more rapidly. Temperature is simply the measurement of this average kinetic energy. When the particles move faster, the temperature we measure goes up.
The critical distinction to grasp is that heat is the transfer of energy, while temperature is the measure of the average molecular motion within a substance. Heating is the cause; a rise in temperature is the most common effect.

Defining the Core Concepts: Heat vs. Temperature
To truly understand why heating increases temperature, we must first be precise about what these two terms mean. They are related, but not interchangeable.
What is Temperature?
Temperature is a measure of the average kinetic energy of the atoms and molecules in a system. Kinetic energy is the energy of motion.
In a solid, this motion is primarily vibration. In a liquid or gas, it includes vibration, rotation, and translation (moving from place to place). A higher temperature means the particles are, on average, moving more energetically.
What is Heat?
Heat is not something an object has; it is energy in transit. Specifically, heat is the flow of thermal energy from a hotter object or area to a colder one.
This transfer occurs because of the temperature difference. The process of adding this energy to a system is what we call "heating."
The Cause-and-Effect Relationship
Heating a substance is the act of transferring energy into it. This added energy increases the total internal energy of the substance's molecules.
This increase in internal energy directly translates to an increase in the kinetic energy of the particles. Since temperature is the measure of that kinetic energy, the temperature rises. Think of it as pushing a child on a swing: the push is the heat (energy transfer), and the higher swing arc is the increased temperature (more motion).
When Heating Doesn't Increase Temperature
Understanding the exceptions to the rule provides deeper insight. Sometimes, you can add heat to a substance without changing its temperature at all. This occurs during a phase change, such as melting ice or boiling water.
The Role of Latent Heat
The energy required to change the state of a substance (e.g., from solid to liquid) is called latent heat.
When ice at 0°C (32°F) absorbs heat, its temperature does not rise. Instead, all the energy is used to break the rigid bonds holding the water molecules in a fixed ice crystal structure.
Where the Energy Goes
During a phase change, the added energy increases the molecules' potential energy, not their kinetic energy. The molecules are being moved farther apart against the forces that hold them together.
Because temperature is a measure of kinetic energy, the temperature remains constant until all the ice has melted into liquid water. Only after the phase change is complete will adding more heat begin to raise the temperature of the liquid water.
Specific Heat Capacity
The amount of energy required to raise the temperature of a substance is determined by its specific heat capacity. Materials like water have a high heat capacity, meaning they require a lot of energy to get hotter. Metals, in contrast, have a low heat capacity and heat up very quickly.
Applying This Understanding
This knowledge helps you interpret the physical world, whether you are cooking a meal or designing a complex engineering system.
- If your primary focus is understanding basic physics: Remember that temperature measures molecular motion, and heat is the energy that causes that motion to increase.
- If your primary focus is practical application (like cooking or engineering): Recognize that a material's specific heat capacity determines how quickly it heats up, while latent heat explains why temperatures stall during melting or boiling.
Ultimately, grasping the link between heat and temperature is about seeing the invisible dance of molecules in everything around us.
Summary Table:
| Concept | Definition | Key Role in Heating |
|---|---|---|
| Heat | Energy in transit from a hotter to a colder object. | The cause; it's the energy being added to the system. |
| Temperature | A measure of the average kinetic energy of particles. | The effect; it quantifies the increased motion from heating. |
| Latent Heat | Energy used to change a substance's state (e.g., melting). | Explains why temperature stays constant during a phase change. |
| Specific Heat Capacity | The energy needed to raise 1 gram of a substance by 1°C. | Determines how quickly a material's temperature will rise. |
Master the principles of thermal science in your lab. Understanding heat transfer is crucial for precise experiments, from material synthesis to chemical analysis. KINTEK specializes in providing reliable lab equipment, including ovens, furnaces, and heating mantles, designed for accurate temperature control and efficient energy transfer. Let our expertise enhance your research—contact us today to find the perfect heating solution for your laboratory's needs.
Visual Guide
Related Products
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Vertical Laboratory Tube Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
People Also Ask
- What is the difference between cast and sintered parts? Choose the Right Metal Forming Process
- Why Use HIP or SPS After Mechanical Alloying of Alloys? Achieve Full Density and Structural Integrity
- What are the two types of hot air ovens? Choose the Right Air Circulation for Your Lab
- How efficient is plastic pyrolysis? Maximizing Waste-to-Energy Conversion
- What is sintered powdered metal? A Guide to Net-Shape Metal Parts
- How does multi-stage drying in a laboratory oven benefit CMC? Optimize Purity and Preserve Chemical Integrity
- What are the limitations of fluidized bed reactor? Key Challenges in Design and Operation
- What is a sintered metal? A Guide to High-Strength, Complex Metal Parts


























