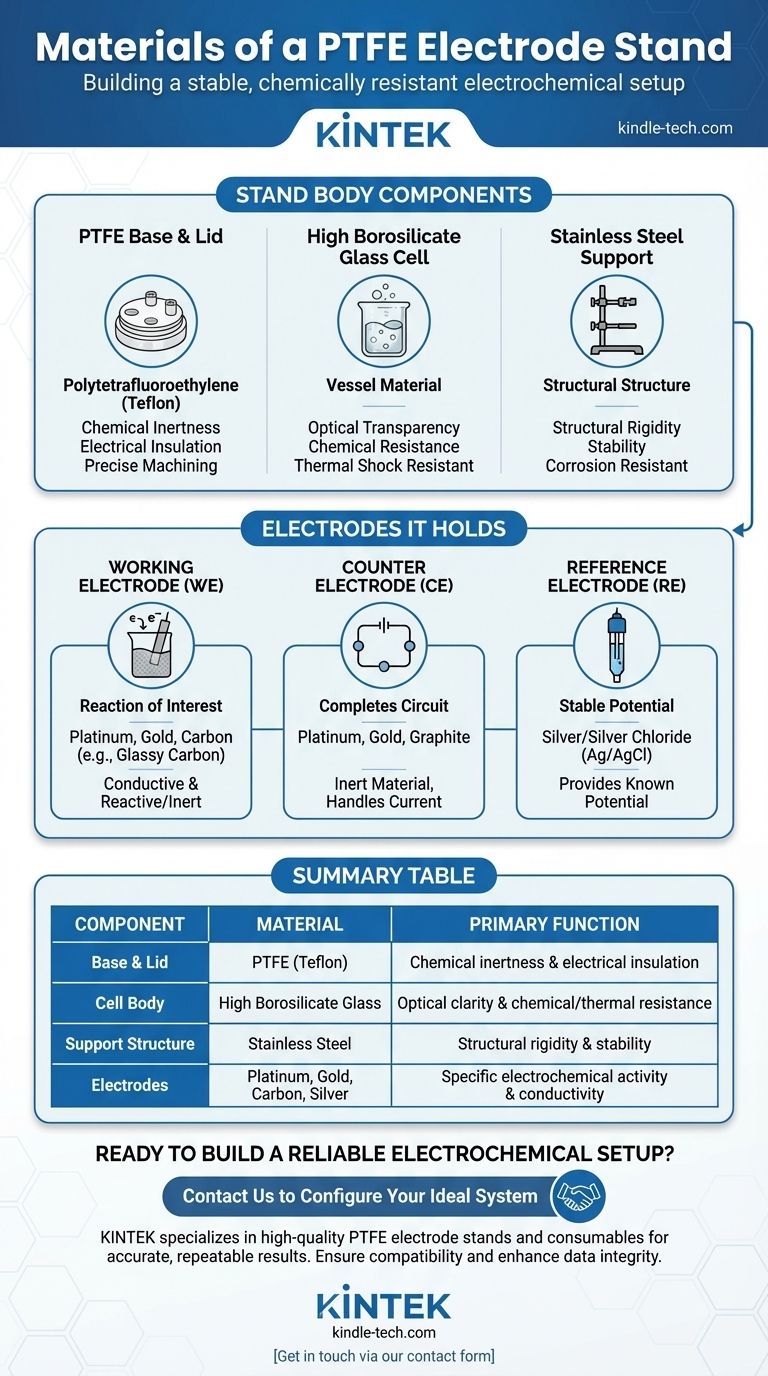
In short, a standard PTFE electrode stand is constructed from a chemically resistant PTFE (Polytetrafluoroethylene) base and lid, a transparent high borosilicate glass cell body, and a sturdy stainless steel support structure. The electrodes held by the stand use a wider range of materials—such as platinum, gold, carbon, and silver—chosen specifically for their electrochemical properties.
The selection of materials for an electrochemical cell is a deliberate balance. The stand provides an inert, stable structure, while the electrodes introduce the specific conductive and reactive properties required for the experiment.

The Core Components of the Stand
The main body of the stand is designed for stability, visibility, and chemical resistance. Each material serves a distinct, non-electrochemical purpose.
The PTFE Base and Lid
Polytetrafluoroethylene (PTFE), commonly known by the brand name Teflon, is used for the base and lid. Its primary benefits are its exceptional chemical inertness and high resistance to a wide range of solvents and electrolytes.
PTFE is also a very good electrical insulator, preventing any unwanted electrical pathways. Its relative softness allows for precise machining of ports and fittings that hold the electrodes securely.
The High Borosilicate Glass Cell
The cell body, or vessel, is typically made from high borosilicate glass. This material offers excellent optical transparency, allowing you to visually monitor the reaction, observe gas evolution, or note color changes.
Like PTFE, it is highly resistant to chemical corrosion and can withstand significant thermal shock, making it suitable for experiments run at various temperatures.
The Stainless Steel Support
The support rod and any associated clamps are made of stainless steel. This material's function is purely structural.
It provides the rigidity and weight needed to keep the entire assembly stable and prevent tipping. Its corrosion resistance is sufficient for a typical laboratory environment, though it is not intended to come into contact with the electrolyte solution.
Understanding the Electrodes It Holds
While the stand itself is inert, the electrodes it holds are the active heart of the electrochemical system. Their materials are chosen based on their role in the reaction.
The Working Electrode (WE)
This is where the reaction of interest occurs. The material must be a good electrical conductor, and it can be either inert (not participating in the reaction) or reactive (acting as a reactant).
Common materials include platinum, gold, and various forms of carbon (like glassy carbon), which are chosen for their conductive properties and stability.
The Counter (or Auxiliary) Electrode (CE)
The counter electrode completes the electrical circuit. It must be made from an inert material that does not react or dissolve in the electrolyte.
Materials like platinum, gold, and graphite are frequently used because they can handle the current without interfering with the primary reaction at the working electrode.
The Reference Electrode (RE)
This electrode provides a stable, known potential against which the working electrode's potential is measured. A typical reference electrode, such as a Silver/Silver Chloride (Ag/AgCl) electrode, has a complex internal structure.
It consists of a silver wire coated in silver chloride, submerged in a filling solution (often potassium chloride), and housed within a glass or plastic body that acts as a salt bridge.
Critical Considerations and Best Practices
Understanding the materials is key to ensuring the integrity and repeatability of your results. Missteps in handling or selection can compromise your entire experiment.
Material Compatibility
Before starting an experiment, always verify that your electrolyte solution and analytes are compatible with all "wetted" parts. This includes the glass cell, the PTFE lid, and the specific materials of all three electrodes.
Proper Cleaning and Maintenance
The surfaces of your cell components are critical. Clean them by wiping with deionized water or a suitable solvent recommended by the manufacturer.
Never use abrasive tools like steel wool or harsh scouring pads. These will create microscopic scratches on the glass and PTFE surfaces, which can trap impurities and ruin future experiments.
The Importance of Inertness
The stand's components (PTFE, glass) are designed to be completely inert to avoid introducing variables into your experiment. Any contamination or unintended side reaction from these parts invalidates the data collected from the carefully chosen electrodes.
Making the Right Choice for Your Experiment
Your experimental goal dictates the specific materials you need.
- If your primary focus is general-purpose voltammetry: The standard setup with a borosilicate glass cell, PTFE lid, and common electrodes (e.g., glassy carbon WE, platinum CE, and Ag/AgCl RE) is a robust starting point.
- If you are working with highly aggressive chemicals (like hydrofluoric acid): You must verify that borosilicate glass is suitable or substitute the cell with a fully PTFE vessel.
- If your experiment involves electrodeposition or stripping: Your working electrode material is a critical reactant or substrate, so its choice (e.g., a mercury drop, bismuth film, or specific metal) is central to your method.
Ultimately, understanding the function of each material empowers you to build a reliable electrochemical system and generate accurate, trustworthy data.
Summary Table:
| Component | Material | Primary Function |
|---|---|---|
| Base & Lid | PTFE (Teflon) | Chemical inertness & electrical insulation |
| Cell Body | High Borosilicate Glass | Optical clarity & chemical/thermal resistance |
| Support Structure | Stainless Steel | Structural rigidity & stability |
| Electrodes | Platinum, Gold, Carbon, Silver | Specific electrochemical activity & conductivity |
Ready to build a reliable electrochemical setup for your lab?
The right materials are the foundation of accurate and repeatable results. KINTEK specializes in high-quality lab equipment and consumables, including PTFE electrode stands and a wide range of electrodes, designed for stability and chemical resistance.
We can help you:
- Select the perfect stand and electrode materials for your specific application.
- Ensure compatibility with aggressive chemicals or specialized techniques.
- Enhance the integrity and reliability of your electrochemical data.
Contact us today to discuss your laboratory needs and let our experts help you configure the ideal system. Get in touch via our contact form to get started!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
People Also Ask
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results



















