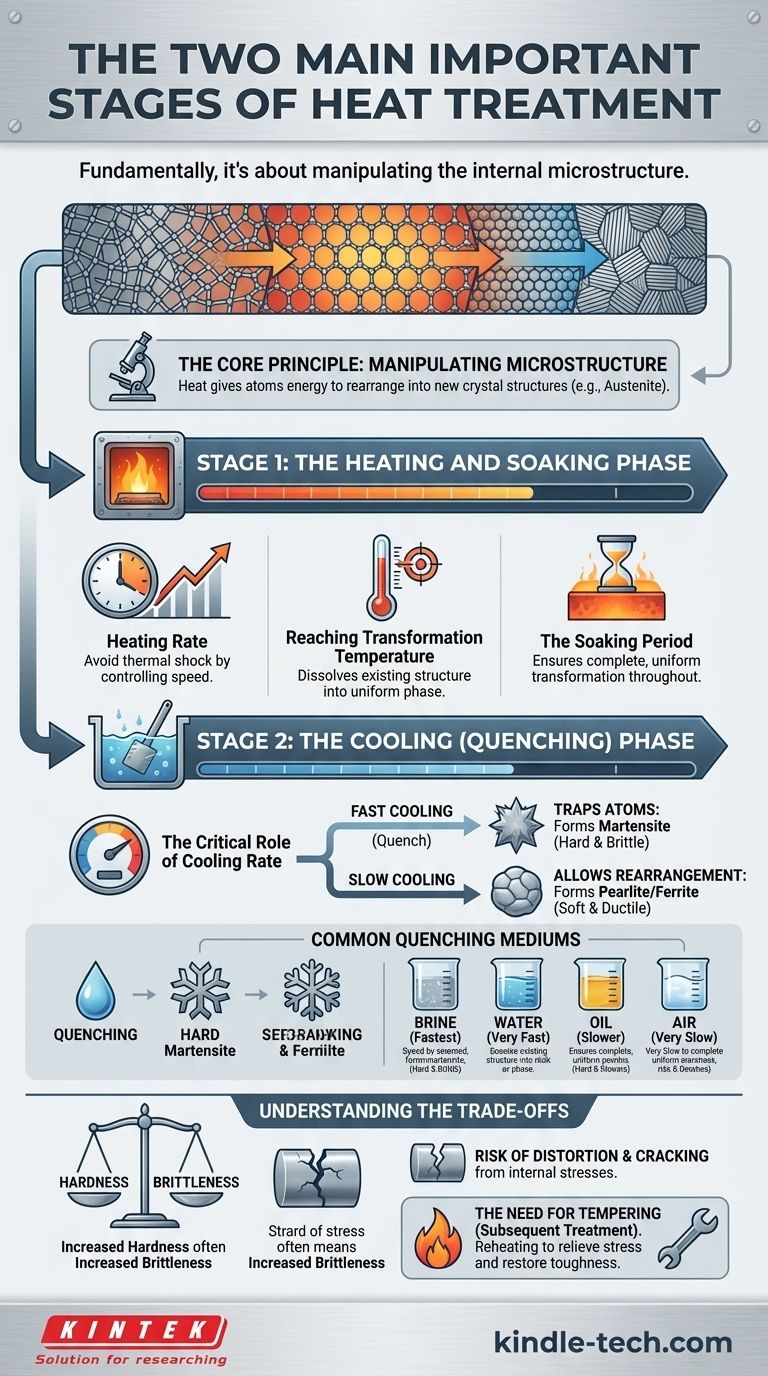
Fundamentally, the heat treatment of metals is a two-part process involving a heating and soaking stage, followed by a controlled cooling stage. The purpose is not merely to heat and cool the material, but to use temperature and time to deliberately change its internal crystalline structure, thereby altering its physical properties like hardness, strength, and ductility.
The two most critical stages are not just actions but controlled phases: the Heating and Soaking Stage, where the material's atomic structure is prepared for change, and the Cooling Stage, which locks in the new, desired microstructure and its corresponding properties.

The Core Principle: Manipulating Microstructure
To understand heat treatment, you must think of metal not as a solid, static block, but as a rigid lattice of atoms. Heat gives these atoms the energy to move and rearrange themselves into different crystal structures.
What is Microstructure?
Microstructure refers to the specific arrangement of these crystals, or "grains," within the metal. Different arrangements result in vastly different material properties.
For steel, a common example, heating it above a critical temperature transforms its structure into a phase called austenite. The final properties of the steel are determined by what this austenite transforms into upon cooling.
Stage 1: The Heating and Soaking Phase
The first stage sets the foundation for the entire process. Its goal is to transform the material into a uniform, high-temperature phase (like austenite) from which the final structure can be formed.
The Heating Rate
The speed at which a part is heated is critical. Heating too quickly can cause thermal shock, creating internal stresses that lead to distortion or cracking, especially in complex shapes or thick sections.
Reaching the Transformation Temperature
Every heat treatment process has a target temperature. For hardening steel, this is the austenitizing temperature. At this point, the existing microstructure dissolves and recrystallizes into the uniform austenite phase, creating a "clean slate."
The Soaking Period
Once at the target temperature, the material is held, or "soaked," for a specific duration. This ensures that the transformation is complete and uniform throughout the entire cross-section of the part, from the surface to the core.
Stage 2: The Cooling (Quenching) Phase
This is arguably the most decisive stage, as the rate of cooling dictates the final microstructure and, therefore, the material's properties.
The Critical Role of Cooling Rate
The speed at which the material is cooled from its transformation temperature determines which new crystal structures can form.
A fast cooling rate, or quench, traps the atoms in a highly stressed, hard structure called martensite. A slow cooling rate, in contrast, allows the atoms to rearrange into softer, more ductile structures like pearlite or ferrite.
Common Quenching Mediums
The cooling rate is controlled by the quenching medium. The choice of medium depends on the material and the desired hardness.
- Brine (salt water): Provides the fastest quench, but with a high risk of distortion.
- Water: A very fast quench, effective but can also cause cracking.
- Oil: A slower quench than water, reducing the risk of cracking while still achieving good hardness.
- Air: A very slow "quench," used in processes like normalizing or for specific "air-hardening" steels.
Understanding the Trade-offs
Heat treatment is not a magic bullet; it is a process of engineered compromises. Understanding these trade-offs is essential for successful application.
Hardness vs. Brittleness
The most fundamental trade-off is between hardness and toughness. Creating a very hard structure like martensite through rapid quenching also makes the material extremely brittle and susceptible to fracture.
The Risk of Distortion and Cracking
Rapid cooling is inherently a violent process. The temperature difference between the surface and the core of a part induces massive internal stresses. These stresses can cause the part to warp, distort, or even crack during or after quenching.
The Need for Subsequent Treatments (Tempering)
Because a fully hardened, as-quenched part is often too brittle for practical use, a secondary heat treatment is almost always required. This process, called tempering, involves reheating the part to a much lower temperature to relieve stress and restore a degree of toughness, albeit at the cost of some hardness.
Matching the Process to the Goal
The correct heat treatment strategy is entirely dependent on the intended function of the component.
- If your primary focus is maximum hardness (e.g., for a cutting tool or bearing): You will use a process that ends with a very rapid quench to form a martensitic structure.
- If your primary focus is softness and ductility (e.g., to prepare a part for extensive machining or forming): You will use an annealing process, which involves very slow cooling inside a furnace.
- If your primary focus is balancing strength and toughness (e.g., for a structural shaft or bolt): You will use a quenching process to harden the part, followed immediately by tempering to reduce brittleness.
By controlling these fundamental stages of heating and cooling, you can engineer a single piece of metal to serve vastly different purposes.
Summary Table:
| Stage | Key Action | Primary Goal |
|---|---|---|
| 1. Heating & Soaking | Heat to target temperature and hold (soak) | Achieve uniform, high-temperature microstructure (e.g., austenite) |
| 2. Cooling (Quenching) | Control the cooling rate (quench) | Lock in the final microstructure and desired material properties |
Ready to achieve precise material properties in your lab?
The two stages of heat treatment are fundamental, but success depends on precise control. KINTEK specializes in the lab equipment and consumables you need for reliable heat treatment processes, from high-temperature furnaces for uniform heating and soaking to the right quenching mediums for controlled cooling.
Let us help you optimize hardness, strength, and ductility for your specific applications. Contact our experts today to discuss your laboratory's heat treatment needs!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
People Also Ask
- What are the uses of vacuum furnace? Achieve Unmatched Material Purity and Performance
- Can I vacuum the inside of my furnace? A Guide to Safe DIY Cleaning vs. Professional Service
- What is the maximum temperature in a vacuum furnace? It Depends on Your Materials and Process Needs
- What is the vacuum heat treatment cycle? Achieve Superior Material Purity and Precision
- What is the standard thickness of plating? Optimize Durability, Corrosion & Cost



















