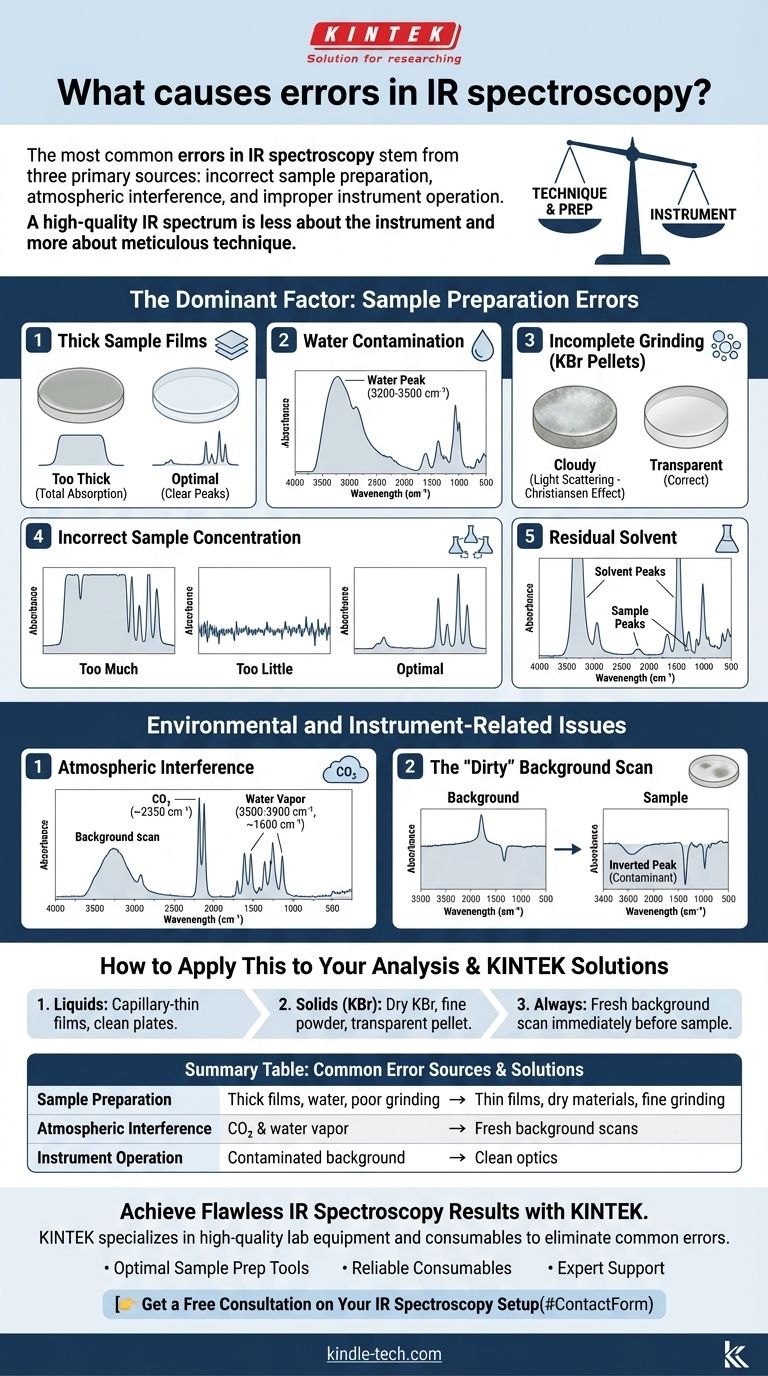
The most common errors in IR spectroscopy stem from three primary sources: incorrect sample preparation, atmospheric interference from carbon dioxide and water, and improper instrument operation, such as running a contaminated background scan. These factors are far more likely to cause a poor result than an actual malfunction of the spectrometer itself.
The core principle to understand is that a high-quality IR spectrum is less about the instrument and more about meticulous technique. Nearly all common errors are preventable by mastering how you prepare and handle your sample before you even press "scan."

The Dominant Factor: Sample Preparation Errors
The way a sample is introduced to the instrument is the single greatest source of error. An improperly prepared sample will produce a spectrum that is misleading or completely uninterpretable, regardless of how advanced the spectrometer is.
Thick Sample Films
If you are analyzing a liquid or a dissolved solid between salt plates, the sample film must be extremely thin.
A sample that is too thick will cause total absorption of the IR beam at certain frequencies. This results in broad, flat-topped peaks that hit 0% transmittance, making it impossible to determine the true peak shape or position.
Water Contamination
Water is a very strong IR absorber and a common contaminant. It can be present in your sample, in solvents, or absorbed by hygroscopic materials like KBr.
This contamination appears as a very broad, prominent peak around 3200-3500 cm⁻¹, which can easily obscure the actual O-H or N-H stretching signals from your sample. Always use dry solvents and materials.
Incomplete Grinding (KBr Pellets)
When preparing a solid sample in a potassium bromide (KBr) pellet, the sample must be ground to a fine, uniform powder.
If particles are too large, they will scatter the IR light instead of absorbing it. This phenomenon, known as the Christiansen effect, causes a distorted, sloping baseline and can make subtle peaks impossible to identify.
Incorrect Sample Concentration
Too much sample leads to the saturated, flat-topped peaks mentioned earlier.
Conversely, too little sample will produce a spectrum with very weak signals and a low signal-to-noise ratio. The resulting peaks may be difficult to distinguish from the baseline noise.
Residual Solvent
If a solid sample was dissolved in a solvent and not dried completely, the solvent will appear in the spectrum.
Strong solvent peaks (e.g., from acetone or ethanol) can easily overwhelm the peaks from your actual sample, leading to incorrect interpretations.
Environmental and Instrument-Related Issues
Even with a perfectly prepared sample, your results can be compromised by the environment in the lab or simple operational missteps.
Atmospheric Interference
The air in the sample compartment contains both carbon dioxide (CO₂) and water vapor, which absorb IR radiation.
CO₂ appears as a characteristic sharp, strong doublet peak around 2350 cm⁻¹. Water vapor appears as a series of many sharp, weak rotational lines, primarily from 3500-3900 cm⁻¹ and around 1600 cm⁻¹.
The "Dirty" Background Scan
An IR spectrometer works by comparing a sample scan to a background scan (of the empty instrument).
If your background scan was run when contaminants (like a solvent residue) were on the salt plates or ATR crystal, those contaminants will appear as inverted or negative peaks in your final sample spectrum. This is a definitive sign of a bad background.
How to Apply This to Your Analysis
Mastering IR spectroscopy is about developing a systematic and clean workflow. The instrument is precise, but it will precisely measure any errors you introduce.
- If your primary focus is analyzing liquids: Ensure your sample forms an almost invisible, capillary-thin film between clean salt plates; too much sample is the most common failure point.
- If your primary focus is analyzing solids with KBr: Use dry KBr, grind the sample and KBr together until they are a flour-like powder, and ensure your pellet is transparent, not cloudy.
- For any analysis: Always run a fresh background scan immediately before your sample to accurately subtract atmospheric CO₂ and water vapor.
By treating sample preparation with the same care as the spectral interpretation, you will consistently produce clean, reliable, and accurate results.
Summary Table:
| Common Error Source | Key Issue | How to Avoid |
|---|---|---|
| Sample Preparation | Thick films, water contamination, poor grinding | Use thin films, dry materials, fine grinding |
| Atmospheric Interference | CO₂ (~2350 cm⁻¹) and water vapor peaks | Run fresh background scans |
| Instrument Operation | Contaminated background scan | Clean optics before background measurement |
Achieve Flawless IR Spectroscopy Results with KINTEK
Struggling with inconsistent IR spectra? The problem is often technique, not your instrument. KINTEK specializes in providing the high-quality lab equipment and consumables you need to eliminate common errors.
We supply:
- Optimal Sample Prep Tools: Precision KBr presses, durable salt plates, and grinding equipment for perfect pellets and films.
- Reliable Consumables: Anhydrous KBr, dry solvents, and cleaning materials to prevent contamination.
- Expert Support: Guidance on best practices for sample handling and instrument operation.
Stop guessing and start producing publication-quality data. Contact our experts today to discuss your specific lab needs and let us help you optimize your IR workflow.
👉 Get a Free Consultation on Your IR Spectroscopy Setup
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Infrared Thermal Imaging Temperature Measurement Double-Sided Coated Germanium Ge Lens
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- What are the advantages of sintered metal? Achieve Strong, Complex Parts Cost-Effectively
- Which lab grown diamond process is best? Focus on Quality, Not the Method
- When using a rotavap Why should you avoid bumping? Prevent Catastrophic Sample Loss and Contamination
- How is the trapping effect utilized to prevent metal aggregation? Optimize Single-Atom Catalyst Synthesis
- Why is it important to match the freezer temperature to storage recommendations? Optimize Food Safety & Energy Use
- What is sintered powdered metal? A Guide to Net-Shape Metal Parts
- What gas is used in sputtering? Optimize Your Thin Film Deposition Process
- How often should you change the oil in a rotary vane vacuum pump? Optimize Your Pump's Performance & Lifespan



















