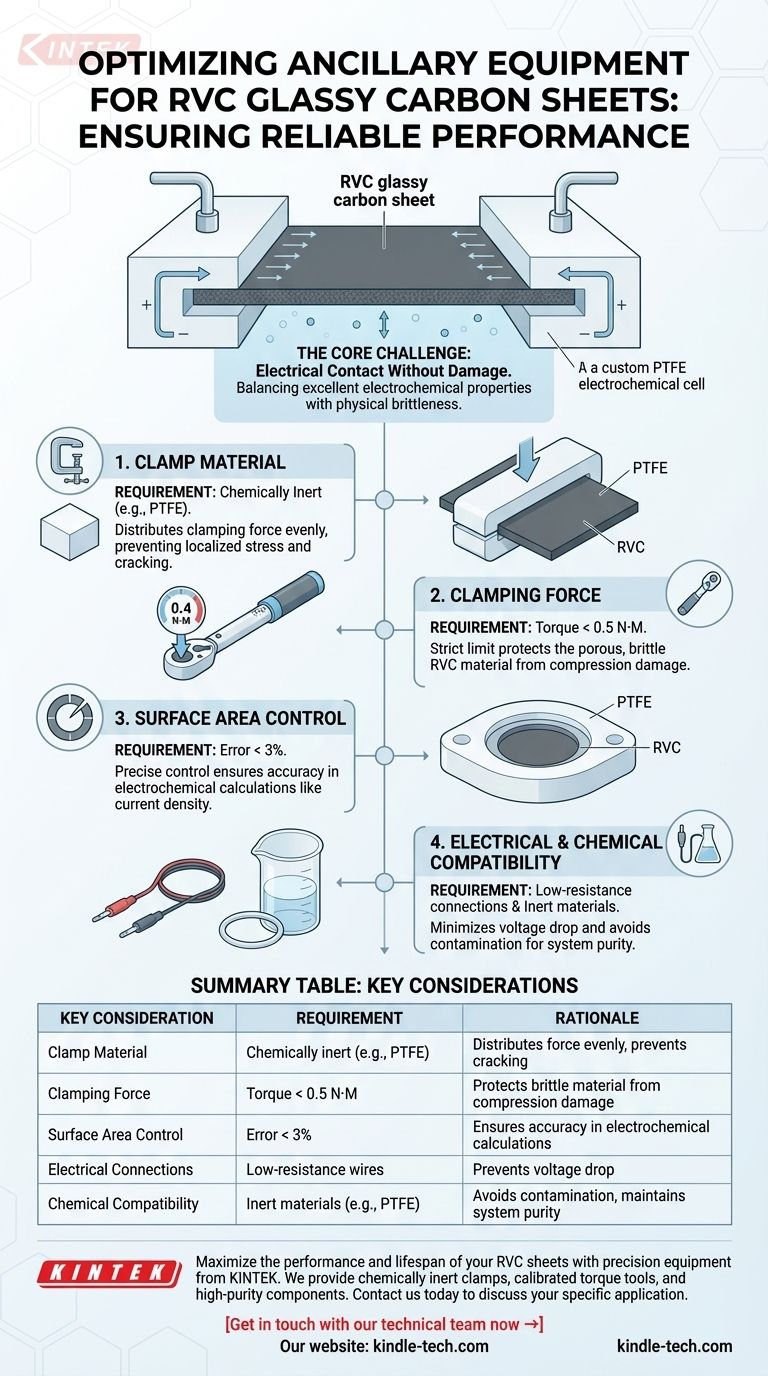
To ensure reliable performance, ancillary equipment for Reticulated Vitreous Carbon (RVC) glassy carbon sheets must be chosen to achieve a stable electrical connection without compromising the material's physical integrity. This involves using chemically inert clamps like PTFE, applying minimal and precise clamping force (torque under 0.5 N·M), and ensuring the exposed surface area is meticulously controlled.
The central challenge when working with RVC sheets is balancing its two defining traits: its excellent electrochemical properties and its inherent physical brittleness. Your ancillary equipment setup must be designed to maximize electrical contact while protecting the material from mechanical stress.

The Core Challenge: Electrical Contact Without Damage
The unique properties of RVC glassy carbon dictate very specific handling and equipment requirements. Its high hardness is paired with low toughness, making it behave much like a ceramic: rigid and strong, but prone to cracking under focused pressure or impact.
Why Clamp Material Matters
The primary point of failure for RVC sheets is cracking due to improper clamping. Using a hard material like a metal clamp creates high-stress concentration points where the clamp meets the sheet, leading to fractures.
To prevent this, a soft, conforming material is required. PTFE (polytetrafluoroethylene) is the standard recommendation because it is soft enough to distribute the clamping force evenly across the surface, preventing localized stress.
The Critical Role of Clamping Force
Even with the correct clamp material, excessive force will damage the sheet. The material's porous, glassy structure cannot withstand high compression.
For this reason, a strict torque limit of no more than 0.5 N·M must be applied. Using a calibrated torque wrench is essential for repeatable and safe installation, removing guesswork and protecting your investment.
Ensuring Accurate Surface Area
RVC's unique microscopic structure provides an exceptionally large specific surface area, which is a key reason for its use in electrochemistry. This also means that small errors in defining the exposed geometric area can lead to significant errors in calculated metrics like current density.
Your setup must allow for precise control of the exposed area, with a recommended error of less than 3%. This is typically achieved using well-machined PTFE or similar holders with precision-cut gaskets or o-rings to define the active region.
Broader System and Environmental Considerations
Beyond the immediate physical clamp, your entire experimental system must be compatible with the RVC sheet to ensure data integrity.
Electrical Connections and Power
RVC possesses good electrical conductivity. To leverage this, your connections must not become the weak link. Use low-resistance wires and connectors to ensure the voltage drop across your ancillary wiring is negligible.
Your power supply or potentiostat must also be stable and capable of delivering the required current without noise, as the high surface area of RVC can support significant electrochemical activity.
Chemical Compatibility
While RVC itself exhibits high chemical stability in a wide range of environments, all other components that come into contact with your electrolyte must do the same.
This reinforces the choice of PTFE for clamps and holders, as it is nearly universally inert. Any gaskets, o-rings, or tubing in the system must also be selected for their compatibility with your specific experimental chemistry to avoid contamination or degradation.
Understanding the Trade-offs and Best Practices
Success with RVC sheets comes from respecting their material properties. Misunderstanding these properties is the most common source of failure.
Brittleness vs. Hardness
Do not confuse hardness with toughness. RVC is very hard and scratch-resistant, but it is also brittle. It will not bend or deform before breaking.
Therefore, you must avoid any bending, squeezing, or sudden impacts. Handle the sheets with care, ideally by the edges, and always ensure they are placed on a flat, stable surface during assembly.
Surface Purity and Contamination
The high surface area and porous structure that make RVC an excellent electrode also make it prone to trapping contaminants.
Always handle the sheets with clean, powder-free gloves. Ensure all ancillary components are thoroughly cleaned before assembly to maintain the purity of the electrochemical system and achieve reproducible results.
Making the Right Choice for Your Setup
Your specific setup will depend on your primary experimental goal. Use these guidelines to prioritize your equipment choices.
- If your primary focus is maximizing experimental accuracy: Prioritize a holder that offers meticulous control over the exposed surface area and use high-quality, low-resistance electrical connections.
- If your primary focus is ensuring material longevity: Strictly adhere to the <0.5 N·M torque limit using a calibrated tool and implement careful handling protocols to prevent any impact or bending.
- If your primary focus is maintaining chemical purity: Ensure every component in contact with the electrolyte, especially clamps and gaskets, is made from a highly inert material like PTFE.
By treating the RVC sheet as a precision component, you can build a reliable experimental setup that delivers accurate and repeatable data.
Summary Table:
| Key Consideration | Requirement | Rationale |
|---|---|---|
| Clamp Material | Chemically inert (e.g., PTFE) | Distributes force evenly, prevents cracking |
| Clamping Force | Torque < 0.5 N·M | Protects brittle material from compression damage |
| Surface Area Control | Error < 3% | Ensures accuracy in electrochemical calculations |
| Electrical Connections | Low-resistance wires/connectors | Prevents voltage drop, leverages RVC's conductivity |
| Chemical Compatibility | Inert materials (e.g., PTFE gaskets) | Avoids contamination, maintains system purity |
Maximize the performance and lifespan of your RVC glassy carbon sheets with precision equipment from KINTEK.
As a specialist in laboratory equipment and consumables, KINTEK understands the critical balance required for working with sensitive materials like RVC. We provide the chemically inert clamps, calibrated torque tools, and high-purity components you need to build a reliable setup that protects your investment and ensures accurate, repeatable data.
Contact us today to discuss your specific application and let our experts help you select the right ancillary equipment for your electrochemical experiments.
Get in touch with our technical team now →
Visual Guide
Related Products
- Platinum Auxiliary Electrode for Laboratory Use
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Li-Air Battery Case for Battery Lab Applications
People Also Ask
- What are the technical advantages of using a spiral platinum wire? Optimize Your Electrochemical Precision
- What is the function of a platinum electrode as an auxiliary electrode? Ensure Precise Nickel Coating Corrosion Testing
- Why is platinum typically selected as the auxiliary electrode for electrochemical testing of oxazoline inhibitors?
- Why is platinum wire selected as the auxiliary electrode? Achieve High-Precision Corrosion Data with Inert Electrodes
- What are the advantages of using a Platinum (Pt) electrode for zirconium testing? Ensure High-Precision Data Integrity



















