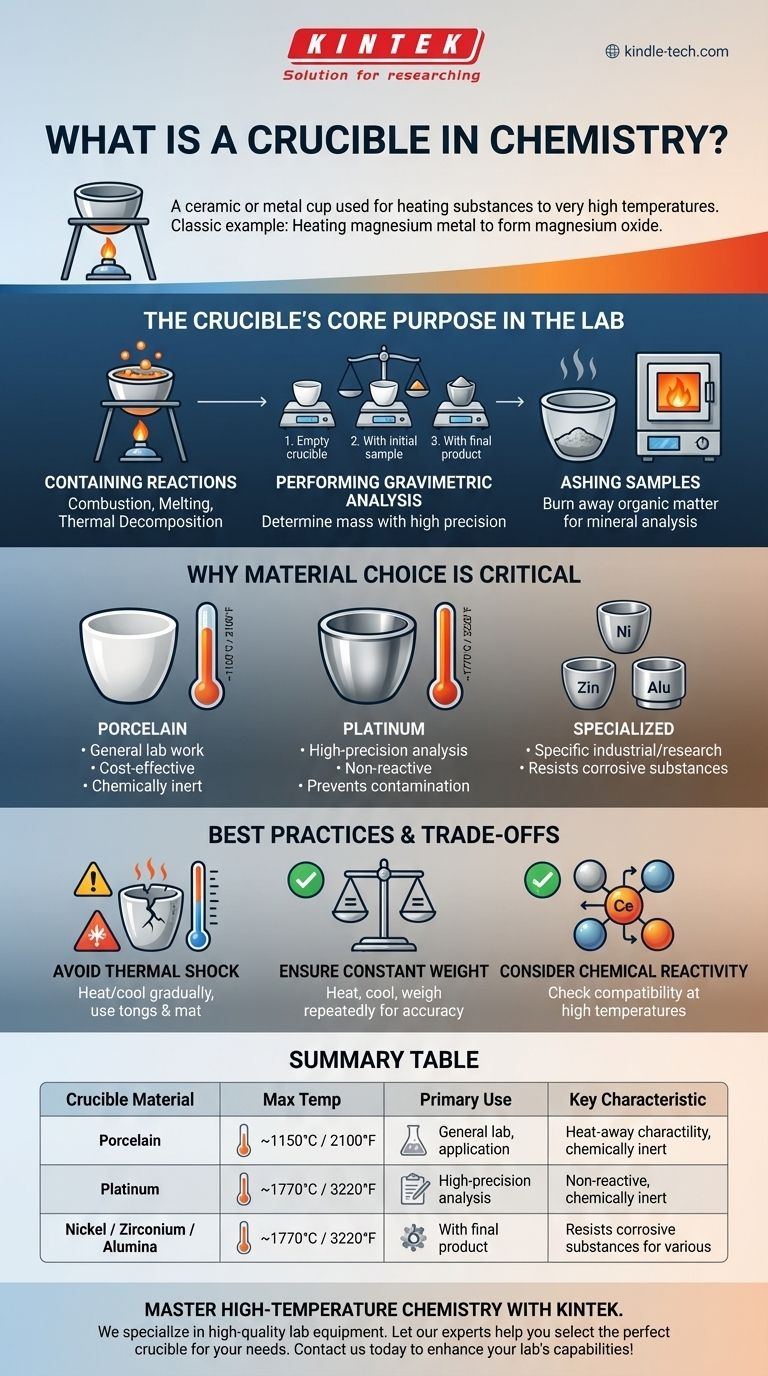
In chemistry, a crucible is a ceramic or metal cup used for heating substances to very high temperatures. A classic example is heating a coil of magnesium metal in a porcelain crucible with a lid, placed over a Bunsen burner. The intense heat causes the magnesium to combust and react with oxygen from the air, forming a white powder, magnesium oxide.
A crucible's primary function is to serve as an inert, heat-resistant container, allowing chemists to perform reactions and analyses at temperatures that would destroy standard glassware. Its significance lies in its thermal and chemical stability under extreme conditions.

The Crucible's Core Purpose in the Lab
A crucible is not just a heat-proof beaker. Its specific design and material properties make it essential for techniques that require extreme heat and precise mass measurements.
Containing High-Temperature Reactions
The most fundamental use of a crucible is to hold chemical samples while they are heated to hundreds or even thousands of degrees Celsius.
This allows for processes like combustion, melting metals, or driving thermal decomposition reactions that require far more energy than a hot plate or water bath can provide.
Performing Gravimetric Analysis
Crucibles are central to gravimetric analysis, a technique used to determine the mass of a substance. A common experiment is finding the empirical formula of a compound like magnesium oxide.
The process involves three key weighings:
- The empty, clean crucible and lid.
- The crucible, lid, and the initial sample (e.g., magnesium).
- The crucible, lid, and the final product after heating (e.g., magnesium oxide).
The difference between these masses allows the chemist to calculate the mass of each element in the final compound with high precision.
Ashing Samples
In food science and environmental analysis, crucibles are used for ashing. A sample (like a piece of food or soil) is heated to a very high temperature to completely burn away all organic matter.
What remains is the inorganic ash (minerals, salts), which can then be weighed or analyzed to determine the sample's mineral content.
Why Material Choice is Critical
The type of crucible used is dictated by the temperature required and the chemical nature of the substances being heated.
Porcelain: The Laboratory Workhorse
White porcelain crucibles are the most common type found in educational and general-purpose labs.
They offer good resistance to high temperatures (up to ~1150°C or 2100°F) and are relatively inexpensive and chemically inert for most common applications.
Platinum: The Standard for High-Precision Analysis
For the most sensitive analytical work, platinum crucibles are used. Platinum has an extremely high melting point (~1770°C or 3220°F) and is exceptionally non-reactive.
This ensures that the crucible itself does not contribute any contaminants to the sample, which is critical when analyzing trace elements.
Specialized Materials: Nickel, Zirconium, and Alumina
For specific industrial or research applications, crucibles are made from other materials. For example, nickel crucibles are used for fusions with strong alkaline substances that would corrode porcelain.
Understanding the Trade-offs and Best Practices
Using a crucible correctly is essential for both safety and accuracy. Mishandling is a common source of error and equipment failure.
Thermal Shock: The Crucible's Weakness
The most common mistake is causing thermal shock. Placing a hot crucible on a cold lab bench or heating it too rapidly can cause it to crack or shatter.
Crucibles must always be heated and cooled gradually. They should be handled only with clean crucible tongs and placed on a pipeclay triangle for heating or a heat-proof mat for cooling.
Ensuring Constant Weight
For accurate gravimetric analysis, the crucible must be heated until it reaches a "constant weight." This means heating and cooling it, then weighing it, and repeating the process until two consecutive weighings are identical.
This procedure guarantees that all volatile impurities, especially absorbed water from the air, have been driven off, providing a true baseline mass.
Chemical Reactivity
While generally inert, no crucible is perfect. At very high temperatures, even porcelain can react with extremely strong bases. A chemist must always consider the potential for a reaction between their sample and the crucible material itself.
Making the Right Choice for Your Goal
Selecting and using a crucible properly depends entirely on the objective of your experiment.
- If your primary focus is a simple school or college demonstration: A standard porcelain crucible is the correct and most cost-effective tool for showing combustion or decomposition.
- If your primary focus is accurate quantitative analysis: You must follow the procedure of heating to a constant weight to ensure your mass measurements are precise and free from moisture-related errors.
- If your primary focus is high-purity research with aggressive chemicals: A specialized platinum or metal crucible is likely required to prevent sample contamination and damage to the equipment.
Ultimately, the crucible is a simple tool that enables complex and precise chemistry by mastering the challenge of extreme heat.
Summary Table:
| Crucible Material | Max Temperature | Primary Use Case | Key Characteristic |
|---|---|---|---|
| Porcelain | ~1150°C (2100°F) | General lab work, education | Cost-effective, chemically inert |
| Platinum | ~1770°C (3220°F) | High-precision analysis | Non-reactive, prevents contamination |
| Nickel / Zirconium / Alumina | Varies | Specialized industrial/research | Resists corrosive substances |
Ready to master high-temperature chemistry with the right crucible?
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your laboratory's needs. Whether you're performing gravimetric analysis, ashing samples, or conducting high-purity research, our selection of crucibles—from durable porcelain to precision platinum—ensures accurate results and long-lasting performance.
Our experts can help you select the perfect crucible material and design for your specific application, ensuring thermal stability and chemical resistance. Let KINTEK be your trusted partner in achieving precise, reliable outcomes in even the most demanding laboratory environments.
Contact us today to discuss your crucible requirements and enhance your lab's capabilities!
Visual Guide
Related Products
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
People Also Ask
- What is the difference between clay graphite crucible and silicon carbide crucible? A Guide to Maximizing Your Melt Efficiency
- What are the disadvantages of a crucible furnace? Understanding Capacity, Cost, and Efficiency Limits
- Why are silica boats or high-purity ceramic crucibles used for Ni-TiO2 sintering? Ensure Pure Catalyst Results
- What material is crucible best made of? Match the Material to Your Metal and Process
- What are the different types of crucible? A Guide to Material, Shape, and Application
- What are the advantages of using graphite crucibles in 3000°C experiments? Achieve Superior Purity and Performance
- How to maintain a crucible? Prevent Thermal Shock and Ensure Longevity
- Does a graphite crucible need to be seasoned? The Critical First-Use Safety Guide



















