In the context of pyrolysis, the heating rate is the speed at which biomass is heated to the target temperature in an oxygen-free environment. Measured in degrees Celsius per second (°C/s), it is one of the most critical parameters in the entire process. This rate directly controls the chemical reactions that occur and, consequently, the final distribution of products.
The pyrolysis heating rate is not a minor technical detail; it is the primary lever used to determine whether the process will yield primarily solid biochar, liquid bio-oil, or combustible gas.

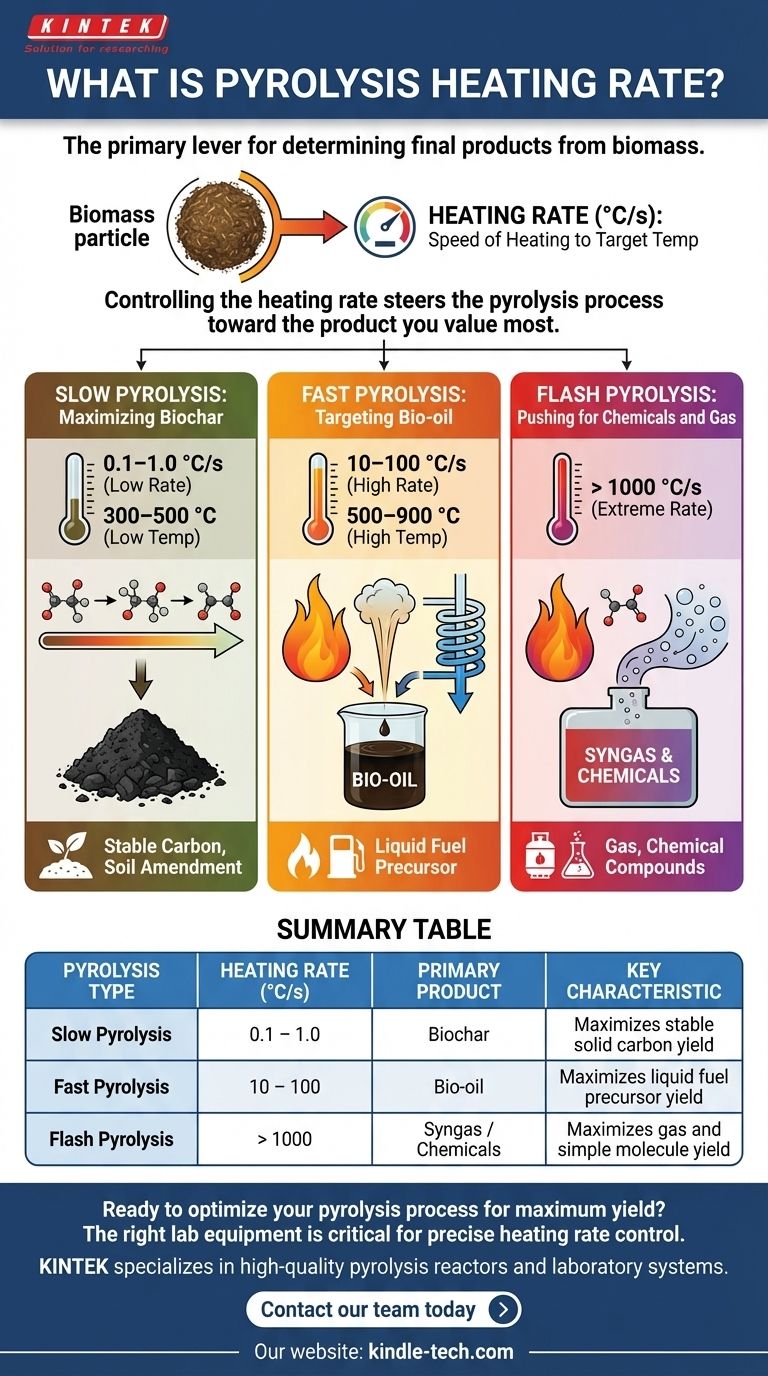
How Heating Rate Dictates Pyrolysis Outcomes
The speed of heating fundamentally alters the reaction pathways. Slower heating allows time for complex molecules to break down and re-form into stable solids, while rapid heating "freezes" them in a vapor state which can be condensed into liquid.
Slow Pyrolysis: Maximizing Biochar
Slow pyrolysis uses very low heating rates, typically between 0.1–1.0 °C/s, and relatively low temperatures (300–500 °C).
This gradual heating process provides ample time for secondary reactions to occur. The initial vapors slowly decompose and repolymerize on the surface of the solid material, maximizing the production of biochar, a stable, carbon-rich solid.
This method has been used for centuries to produce charcoal for fuel and, more recently, for agricultural soil amendment and carbon sequestration.
Fast Pyrolysis: Targeting Bio-oil
Fast pyrolysis employs significantly higher heating rates, generally between 10–100 °C/s, and higher temperatures (500–900 °C). The goal is to heat the biomass particles as quickly as possible.
This rapid energy transfer quickly breaks down the biomass into vapors and aerosols. The vapors are then immediately removed from the hot zone and rapidly quenched (cooled) to prevent further reactions.
This process minimizes char formation and maximizes the yield of a dark, viscous liquid known as bio-oil or pyrolysis oil, which can be a potential source for biofuels and chemicals.
Flash Pyrolysis: Pushing for Chemicals and Gas
Flash pyrolysis represents the extreme end of the spectrum, with heating rates exceeding 1000 °C/s.
This near-instantaneous heating, combined with very short vapor residence times, is designed to crack the biomass molecules into the simplest possible components.
The primary goal is often to maximize the production of combustible gases (syngas) or specific high-value chemical compounds, rather than liquid or solid yields.
Understanding the Trade-offs
Choosing a heating rate is an engineering decision with significant consequences for product quality and process complexity. The "best" method depends entirely on the desired outcome.
The Challenge of Bio-oil Quality
While fast pyrolysis maximizes liquid yield, the resulting bio-oil is not a direct replacement for crude oil.
It has a very high oxygen content, which makes it acidic, corrosive, and thermally unstable. It also does not mix well with conventional fossil fuels and is prone to thickening or solidifying over time. Upgrading this oil is a significant technical challenge.
Engineering Complexity and Cost
Slow pyrolysis can be achieved with relatively simple and robust technology, such as a basic kiln.
In contrast, achieving the high heat transfer rates required for fast and flash pyrolysis demands highly sophisticated reactors, such as fluidized beds or ablative systems. These systems are more complex, expensive to build, and more sensitive to operate.
Making the Right Choice for Your Goal
Controlling the heating rate is how you steer the pyrolysis process toward the product you value most. The optimal rate is defined by your specific objective.
- If your primary focus is soil amendment or carbon sequestration: Use slow pyrolysis, as its low heating rate is specifically designed to maximize stable biochar yield.
- If your primary focus is creating a liquid fuel precursor: Use fast pyrolysis, as its high heating rate and rapid quenching maximize the conversion of biomass into bio-oil.
- If your primary focus is producing syngas or specific chemical compounds: Use flash pyrolysis, as its extreme heating rates favor the cracking of vapors into gases and simple molecules.
Ultimately, mastering the heating rate is fundamental to unlocking the specific value you seek from biomass.
Summary Table:
| Pyrolysis Type | Heating Rate Range (°C/s) | Primary Product | Key Characteristic |
|---|---|---|---|
| Slow Pyrolysis | 0.1 – 1.0 | Biochar | Maximizes stable solid carbon yield |
| Fast Pyrolysis | 10 – 100 | Bio-oil | Maximizes liquid fuel precursor yield |
| Flash Pyrolysis | > 1000 | Syngas / Chemicals | Maximizes gas and simple molecule yield |
Ready to optimize your pyrolysis process for maximum yield? The right lab equipment is critical for precise heating rate control. KINTEK specializes in high-quality pyrolysis reactors and laboratory systems designed to deliver the exact thermal conditions you need. Whether your goal is biochar, bio-oil, or syngas production, our experts can help you select the ideal setup for your research or pilot project. Contact our team today to discuss your specific application and process requirements!
Visual Guide
Related Products
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What is a rotary kiln reactor? A Guide to Industrial Thermal Processing
- What is the drying zone in a rotary kiln? Boost Efficiency with Modern Drying Solutions
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- How does a rotary extractor work? Master Continuous High-Volume Solid Processing
- How are composites processed using sintering? Engineered Material Solutions Through Advanced Thermal Bonding



















