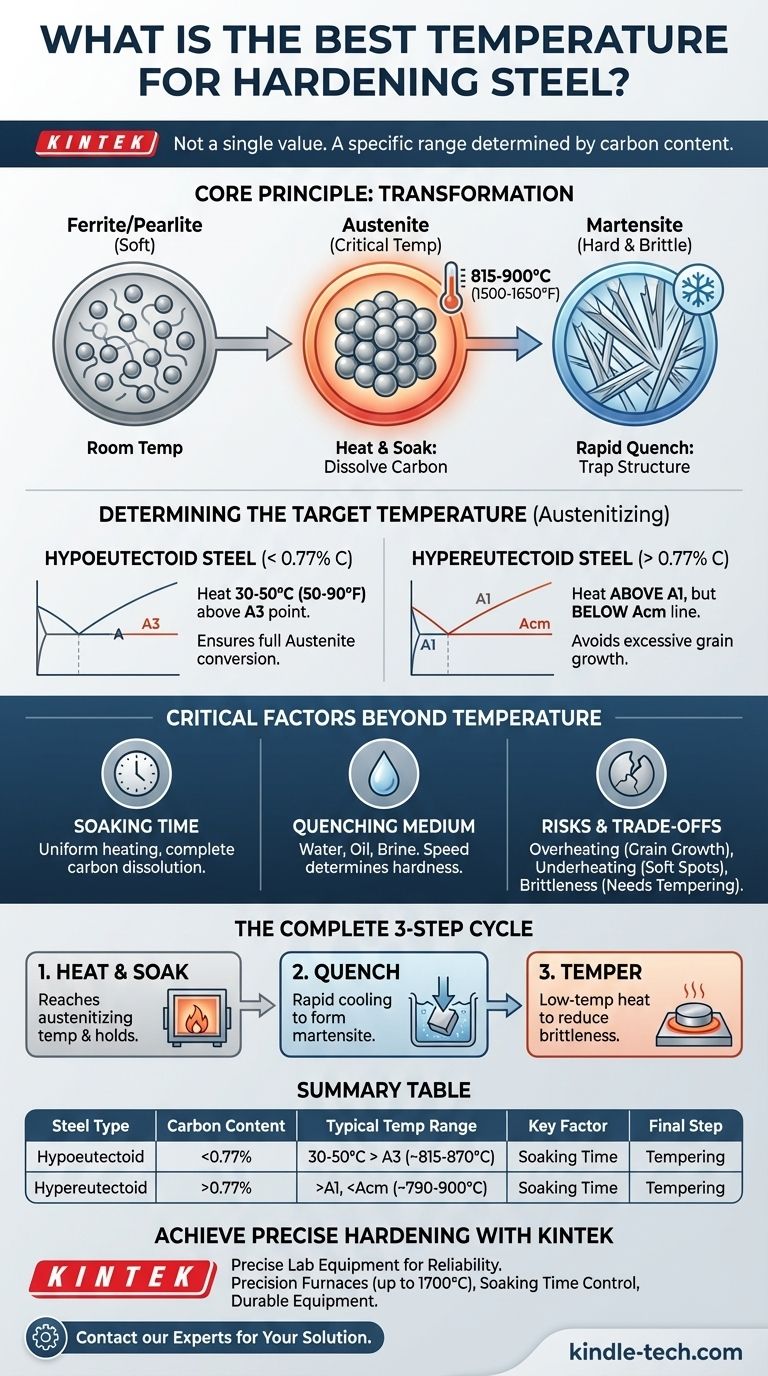
The best temperature for hardening steel is not a single value; it is a specific temperature range determined entirely by the steel's chemical composition, primarily its carbon content. For most common carbon steels, the target temperature—known as the austenitizing temperature—is typically 30-50°C (50-90°F) above its upper critical temperature, placing it in the range of 815-900°C (1500-1650°F). Heating to this precise point is the essential first step in transforming soft steel into a hardened state.
The core principle of hardening is not about achieving a generic "hot" temperature. It's about heating a specific steel alloy above its unique transformation point to create a new internal structure called austenite, then rapidly cooling (quenching) to trap that structure as an intensely hard but brittle phase known as martensite.

The "Why" Behind Hardening: Understanding Steel's Transformation
To control the hardening process, you must first understand what is happening inside the steel. Steel is not a static material; its internal crystal structure changes dramatically with temperature.
From Ferrite to Austenite
At room temperature, steel exists in a relatively soft, ductile state with a crystal structure known as ferrite or a mix of ferrite and iron carbide called pearlite. This structure can only hold a very small amount of carbon in solution.
When you heat the steel, you supply the energy needed for its atoms to rearrange.
The Critical Transformation Temperature
As the steel heats up, it reaches a critical temperature where its crystal structure fundamentally changes. It transforms from ferrite into a new phase called austenite.
This transformation is the secret to hardening. The austenitic crystal structure is capable of dissolving a significant amount of carbon, much like how hot water can dissolve more sugar than cold water. This critical temperature is denoted as A3 for lower-carbon steels and Acm for higher-carbon steels.
The Role of Carbon Content
The exact temperature at which this transformation occurs is dictated almost entirely by the amount of carbon in the steel.
This is why there is no single "best" temperature. A low-carbon steel like 1018 will have a different critical temperature than a high-carbon steel like 1095. This relationship is mapped out on a technical chart called the Iron-Carbon Phase Diagram.
Determining the Correct Hardening Temperature
The goal is to heat the steel just enough to fully convert its structure to austenite, allowing all the carbon to go into solution.
For Steels Under 0.77% Carbon (Hypoeutectoid)
For these common steels, you must heat the material completely above the A3 critical temperature.
A reliable rule of thumb is to identify the steel's A3 point and then add 30-50°C (50-90°F). This ensures a complete and uniform austenitic structure, ready for quenching.
For Steels Over 0.77% Carbon (Hypereutectoid)
For high-carbon tool steels, the approach is different. You heat the steel above the lower critical temperature (A1) but often below the upper Acm line.
Heating these steels too high can cause excessive grain growth and failure to convert all the austenite during the quench, leading to a brittle and less effective final product.
Critical Factors Beyond Temperature
Reaching the right temperature is only the first step. To achieve successful hardening, two other factors are equally important.
The Importance of Soaking Time
The steel must be held at the austenitizing temperature for a specific period, known as soaking.
Soaking ensures that the temperature is uniform throughout the entire part—from the surface to the core—and gives the carbon enough time to dissolve completely into the austenite. Thicker parts require significantly longer soaking times.
The Quench: Trapping the Hardness
Once the steel is properly soaked, it must be cooled rapidly in a process called quenching.
This rapid cooling does not give the austenitic structure time to revert to its soft, room-temperature state. Instead, it traps the dissolved carbon atoms, forcing the creation of the hard, needle-like structure called martensite. The speed of the quench is critical.
The Quenching Medium
The liquid used for quenching—such as water, brine, oil, or even air for certain alloy steels—is chosen based on the steel's hardenability. Using the wrong quenchant can cool the part too slowly (failing to harden it) or too quickly (causing it to crack or warp).
Understanding the Trade-offs and Risks
Precision is essential in heat treatment because small deviations can lead to complete failure.
The Risk of Overheating
Heating steel far above its required austenitizing temperature is a common and irreversible error. It causes the internal grains of the steel to grow excessively, making the final product permanently coarse and brittle, even after quenching and tempering.
The Problem of Underheating
Failing to reach the full austenitizing temperature means the conversion to austenite will be incomplete. The result is a part with soft spots and an inability to achieve the desired hardness and wear resistance.
The Brittleness of Martensite
It is crucial to understand that a fully hardened, as-quenched part is almost always too brittle for practical use. It has maximum hardness but zero toughness. This is why hardening is never the final step in the process.
Making the Right Choice for Your Goal
Successful hardening requires seeing the process as a complete cycle, not just a single temperature target. The final, non-negotiable step is tempering—a low-temperature heat treatment performed immediately after quenching to reduce brittleness and impart toughness.
- If your primary focus is working with a known steel (e.g., 1084, 5160, O1): Your first step should be to consult the manufacturer's or supplier's data sheet. It will provide the precise recommended austenitizing temperature range for that specific alloy.
- If your primary focus is working with unknown carbon steel: A magnet can provide a rough guide. Steel loses its magnetism as it approaches its critical temperature. Heat the steel until a magnet no longer sticks, and then heat it slightly hotter (a dull cherry red to orange) to ensure you are fully in the austenitic range.
- If your primary focus is achieving a reliable outcome: Always remember the complete three-step process for creating a strong, usable part: 1. Heat to the correct austenitizing temperature and soak, 2. Quench in the appropriate medium to form martensite, and 3. Temper immediately to achieve the final desired balance of hardness and toughness.
Mastering steel hardening lies not in finding a single number, but in understanding and controlling the full thermal transformation for your specific material and goal.
Summary Table:
| Steel Type | Carbon Content | Typical Austenitizing Temperature Range |
|---|---|---|
| Hypoeutectoid Steels | < 0.77% | 30-50°C (50-90°F) above A3 point (~815-870°C) |
| Hypereutectoid Steels | > 0.77% | Above A1 point but below Acm line (~790-900°C) |
| Key Factor | Soaking Time | Ensures uniform temperature & carbon dissolution |
| Final Step | Tempering | Reduces brittleness after quenching |
Achieve Precise Hardening Results with KINTEK
Mastering the exact temperature for hardening steel is critical for achieving the desired hardness, wear resistance, and toughness in your components. Inconsistent heat treatment can lead to soft spots, warping, or catastrophic brittleness.
KINTEK specializes in the precise lab equipment you need to control every step of the heat treatment process. From high-temperature muffle furnaces for accurate austenitizing to controlled atmosphere ovens for tempering, our solutions are designed for reliability and repeatability.
We help our laboratory and manufacturing customers by providing:
- Precision Furnaces: For exact temperature control up to 1700°C.
- Soaking Time Control: Ensuring uniform heat treatment throughout your parts.
- Durable Equipment: Built to withstand the rigors of repeated hardening cycles.
Don't leave your results to chance. Let KINTEK's expertise in lab equipment ensure your hardening process is a success.
Contact our thermal processing experts today to discuss your specific steel hardening requirements and find the perfect solution for your lab.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the difference between furnace and muffle furnace? The Key is Isolation for Purity & Precision
- What is the temperature for a furnace? It Depends on Your Material and Process Goal
- What is the difference between a lab oven and a muffle furnace? A Guide to Temperature Applications
- Which gas is used in a muffle furnace? Choosing the Right Atmosphere for Your Lab Process
- How is heat transferred in a furnace? Master Radiation, Convection & Conduction



















