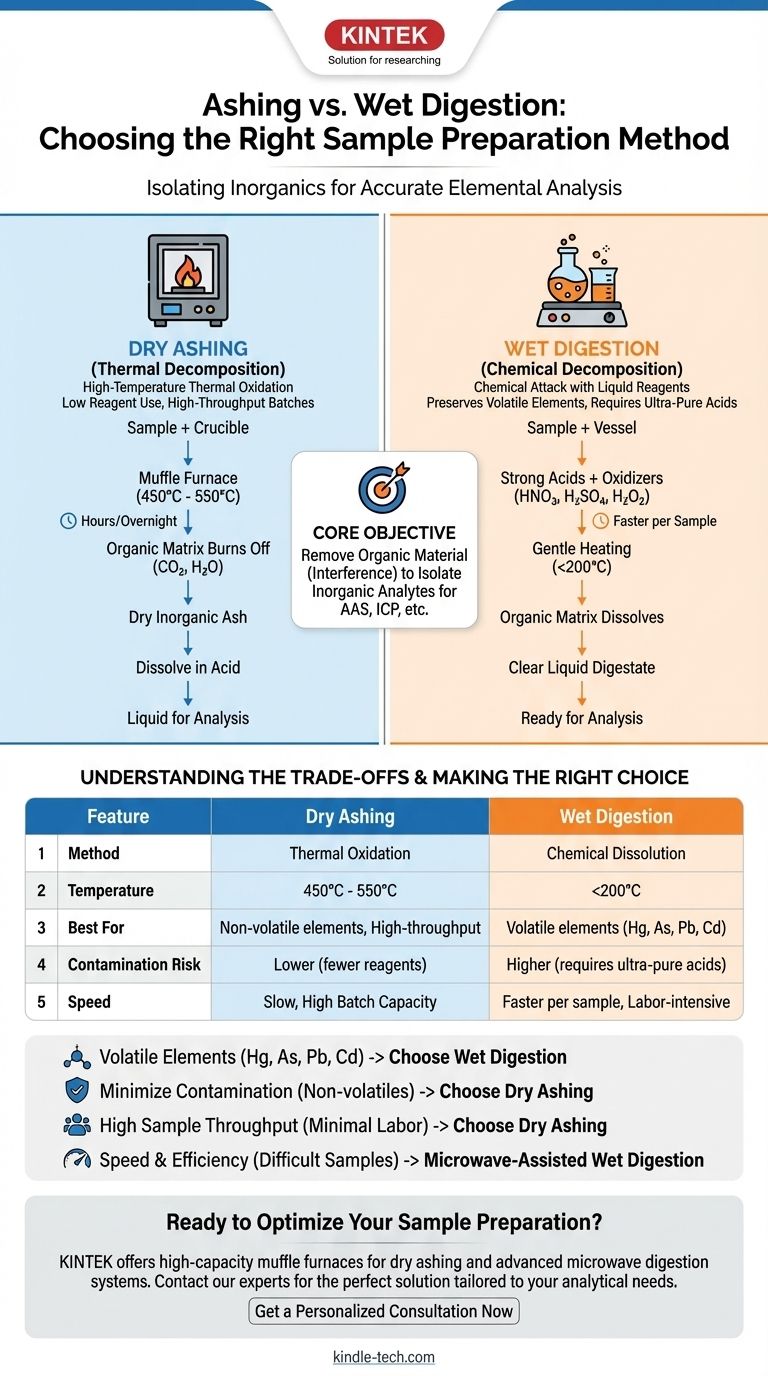
In analytical chemistry, the primary difference between these two sample preparation techniques lies in the method of decomposition. Dry ashing uses high-temperature thermal oxidation in a furnace to burn away the organic matrix, while wet digestion uses liquid chemical reagents, such as strong acids and oxidizing agents, to dissolve it.
The core objective of both methods is the same: to remove organic material that interferes with elemental analysis. Your choice between dry ashing and wet digestion is a critical decision based on the specific elements you need to measure, the risk of contamination, and the nature of your sample.

The Goal: Isolating Inorganics for Analysis
Why Sample Preparation is Necessary
Before you can measure the concentration of specific elements (like lead, iron, or calcium) in a sample, you must first remove the complex organic matrix.
Materials like food, tissue, or plastics are primarily made of carbon, hydrogen, and oxygen. These organic compounds create interference in analytical instruments like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP), making it impossible to get an accurate reading of the trace inorganic elements.
Two Paths to the Same Goal
Both ashing and digestion are destructive methods designed to eliminate this organic background, leaving behind only the inorganic components (the "analytes") for measurement. The fundamental difference is how they achieve this destruction.
Understanding Dry Ashing (Thermal Decomposition)
The Core Process
Dry ashing is conceptually straightforward. The sample is placed in a crucible, typically made of porcelain or quartz, and heated in a muffle furnace at very high temperatures, usually between 450°C and 550°C.
The combination of intense heat and an oxygen-rich atmosphere effectively burns away the organic matter, converting it to carbon dioxide and water vapor.
What Remains
The process leaves behind a small amount of dry, inorganic ash. This ash, which contains the metallic and mineral elements of interest, is then dissolved in acid to create a liquid solution ready for analysis.
Understanding Wet Digestion (Chemical Decomposition)
The Core Process
Wet digestion, also known as acid digestion, uses a chemical attack rather than extreme heat. The sample is placed in a flask or vessel with a cocktail of powerful liquid reagents.
These reagents are typically strong acids (like nitric acid and sulfuric acid) and sometimes oxidizing agents (like hydrogen peroxide or perchloric acid).
The Role of Heat
The mixture is then gently heated, usually well below 200°C. This moderate heat accelerates the chemical reactions, allowing the acids to break down and dissolve the organic matrix, liberating the inorganic elements into the aqueous solution. The entire process results in a clear liquid digestate.
Understanding the Trade-offs
Neither method is universally superior. The choice involves critical trade-offs that directly impact the accuracy of your results.
Analyte Volatility
This is the single most important factor. The high temperatures of dry ashing can cause volatile elements to be lost to the atmosphere before they can be measured. Elements like mercury (Hg), arsenic (As), lead (Pb), and cadmium (Cd) are particularly susceptible.
Wet digestion, with its much lower operating temperatures, is the required method for preserving and accurately measuring these volatile analytes.
Contamination Risk
Dry ashing uses very few reagents (typically just one acid at the final dissolving step), significantly lowering the risk of introducing trace contaminants. The furnace itself is the main potential source of contamination.
Wet digestion requires large volumes of multiple acids. If these acids are not of sufficiently high purity, they can introduce the very elements you are trying to measure, leading to falsely high results. Using ultra-pure acids is essential but costly.
Speed and Throughput
Dry ashing is a slow process, often requiring many hours or even running overnight. However, a large furnace can process dozens of samples simultaneously with very little hands-on labor, making it excellent for high-throughput batches.
Wet digestion is generally faster per sample, especially with modern microwave digestion systems that can complete the process in under an hour. However, it is often more labor-intensive and may have a lower simultaneous sample capacity.
Safety Considerations
Both methods have significant safety risks. Dry ashing involves extreme temperatures, creating a severe burn hazard. Wet digestion requires handling highly corrosive acids that can cause chemical burns and produce toxic fumes. Special care must be taken with perchloric acid, which can be explosive under certain conditions.
Making the Right Choice for Your Analysis
Your decision must be guided by your analytical goal.
- If your primary focus is analyzing for volatile elements (e.g., Hg, As, Pb, Cd): Choose wet digestion to prevent significant analyte loss at high temperatures.
- If your primary focus is minimizing reagent-based contamination for non-volatile elements: Dry ashing is a simple, clean, and effective choice.
- If your primary focus is processing a large number of samples with minimal labor: Dry ashing allows for unattended, high-throughput batch processing.
- If your primary focus is speed and efficiency for difficult-to-dissolve samples: Microwave-assisted wet digestion provides the fastest and most powerful decomposition.
Ultimately, the best preparation method is the one that reliably preserves your specific analytes of interest while efficiently and safely eliminating the sample matrix.
Summary Table:
| Feature | Dry Ashing | Wet Digestion |
|---|---|---|
| Method | Thermal oxidation (high heat) | Chemical dissolution (acids) |
| Temperature | 450°C - 550°C | Typically < 200°C |
| Best For | Non-volatile elements, high-throughput | Volatile elements (Hg, As, Pb, Cd) |
| Contamination Risk | Lower (fewer reagents) | Higher (requires ultra-pure acids) |
| Speed | Slow (hours/overnight), but high batch capacity | Faster per sample, but often more labor-intensive |
Ready to Optimize Your Sample Preparation?
Choosing the right method is critical for accurate elemental analysis. The KINTEK team can help you select the ideal equipment—from high-capacity muffle furnaces for dry ashing to advanced microwave digestion systems for wet digestion—to ensure your lab achieves precise, reliable results.
We specialize in providing robust, reliable lab equipment and consumables tailored to your analytical chemistry needs. Contact our experts today to discuss your application and find the perfect solution for your laboratory.
Get a Personalized Consultation Now
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the temperature accuracy of a muffle furnace? Achieve Precise and Uniform Heating
- What is the difference between a box furnace and a muffle furnace? Choose the Right Lab Furnace for Your Application
- What is the principle working and use of muffle furnace? Achieve Precise, Contamination-Free Heating
- What are the precautions of muffle furnace? Essential Safety Protocols for Your Lab
- How to use a muffle furnace? A Step-by-Step Guide to Safe and Effective Operation



















