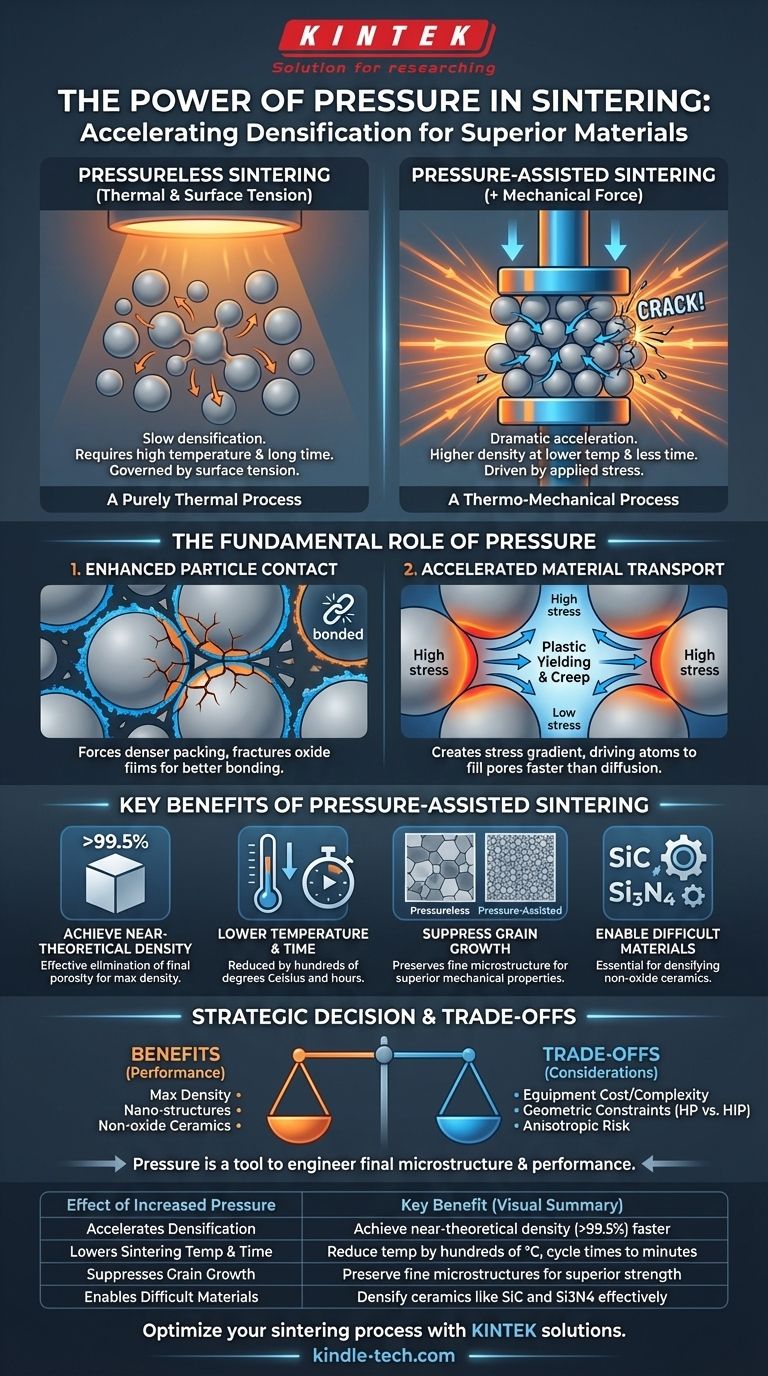
In short, increasing the pressure during sintering dramatically accelerates the densification process, allowing you to achieve a higher final density at a lower temperature and in less time. This applied force acts as a powerful secondary driver for consolidation, supplementing the surface tension effects that govern conventional pressureless sintering.
Applying external pressure is not merely an adjustment; it is a fundamental change to the driving force of sintering. By shifting from a purely thermal process to a thermo-mechanical one, you gain the ability to create denser, stronger materials with finer microstructures that are often impossible to achieve otherwise.

The Fundamental Role of Pressure in Sintering
To understand the effect of pressure, we must first compare it to conventional sintering, which relies solely on thermal energy and surface tension. Pressure introduces a new, dominant force into the system.
From Surface Tension to Mechanical Force
In conventional sintering, atoms slowly move (diffuse) to reduce the total surface energy of the powder compact, causing pores to shrink and particles to bond. This process is driven by heat and is often slow.
Applying external pressure creates high stress at the contact points between particles. This stress provides a powerful mechanical driving force that physically deforms particles and accelerates the very same atomic diffusion mechanisms, but far more effectively.
Enhancing Particle-to-Particle Contact
Pressure forces powder particles into a much denser packing arrangement than is possible through simple settling. This dramatically increases the number and area of contact points between particles.
Critically, this force can fracture brittle surface films (like oxides) that often inhibit atomic diffusion. This exposes clean, reactive surfaces that bond much more readily.
Accelerating Material Transport
The high stress created by pressure generates a stress gradient between the particle contact points and the surfaces of nearby pores. Atoms are actively driven to move from the high-stress contact areas to the low-stress pore surfaces, rapidly filling the voids.
This process, known as plastic yielding and power-law creep, is a much faster material transport mechanism than the surface or grain boundary diffusion that dominates early-stage conventional sintering.
Key Benefits of Pressure-Assisted Sintering
Leveraging pressure is a strategic decision to achieve specific material properties. The primary benefits are significant improvements in density, microstructure, and processing efficiency.
Achieving Near-Theoretical Density
The most significant benefit is the ability to achieve extremely high relative density, often greater than 99.5%. The applied pressure is highly effective at eliminating the final, persistent porosity that is difficult to remove with conventional methods.
Lowering Sintering Temperature and Time
Because pressure provides a strong driving force for densification, you can achieve the target density at a significantly lower temperature. A typical reduction is several hundred degrees Celsius.
This also means the required holding time at peak temperature is much shorter, reducing overall cycle time from many hours to sometimes less than one hour.
Suppressing Grain Growth
Mechanical properties like hardness and strength are heavily dependent on grain size; smaller is generally better. Because pressure allows for lower temperatures and shorter times, it inherently suppresses grain growth. This results in a final product with a fine-grained microstructure and superior mechanical performance.
Sintering Difficult Materials
Many advanced materials, particularly non-oxide ceramics like silicon carbide (SiC) or silicon nitride (Si3N4), have very low intrinsic diffusion rates. They are practically impossible to densify to a high degree using conventional sintering. For these materials, applying pressure is not just an enhancement—it is a necessity.
Understanding the Trade-offs and Limitations
While powerful, pressure-assisted sintering techniques like Hot Pressing (HP) or Hot Isostatic Pressing (HIP) are not a universal solution. You must consider the significant trade-offs.
Equipment Complexity and Cost
The machinery required to apply high pressure at high temperatures is vastly more complex and expensive than a standard furnace. The initial capital investment and ongoing maintenance costs are a primary consideration.
Geometric Constraints
Uniaxial hot pressing, where pressure is applied in one direction, is typically limited to producing parts with simple geometries like discs, blocks, or cylinders. Complex, near-net-shape parts are not feasible.
While Hot Isostatic Pressing (HIP) uses gas pressure to consolidate parts from all directions and allows for more complex shapes, it introduces its own set of design and tooling challenges.
Risk of Anisotropic Properties
In uniaxial hot pressing, the directional pressure can cause an alignment of elongated grains or reinforcing phases. This results in anisotropic properties, where the material's strength and toughness are different when measured parallel versus perpendicular to the pressing direction.
Making the Right Choice for Your Goal
Selecting the right sintering process requires aligning the technique's capabilities with your end goal. Pressure is a tool to be used strategically.
- If your primary focus is achieving maximum density and strength: Use high pressure to eliminate residual porosity, especially for performance-critical ceramic or metal components.
- If your primary focus is processing nano-structured materials: Use pressure to enable low-temperature sintering, which is essential for preserving the nanoscale grain structure.
- If your primary focus is cost-effective, high-volume production of simple parts: Conventional pressureless sintering is likely more economical if the absolute highest performance is not required.
- If your primary focus is consolidating non-oxide ceramics or composites: Pressure is often not a choice but a mandatory requirement to achieve adequate densification.
Ultimately, understanding the role of pressure allows you to move beyond simply heating a material and start truly engineering its final microstructure and performance.
Summary Table:
| Effect of Increased Pressure | Key Benefit |
|---|---|
| Accelerates Densification | Achieve near-theoretical density (>99.5%) faster |
| Lowers Sintering Temperature | Reduce temperature by hundreds of degrees Celsius |
| Suppresses Grain Growth | Preserve fine microstructures for superior strength |
| Enables Sintering of Difficult Materials | Densify ceramics like SiC and Si3N4 effectively |
| Reduces Processing Time | Shorten cycle times from hours to minutes |
Ready to engineer superior materials with precision sintering? At KINTEK, we specialize in advanced lab equipment and consumables tailored for pressure-assisted sintering techniques like Hot Pressing (HP) and Hot Isostatic Pressing (HIP). Whether you're working with high-performance ceramics, composites, or nanostructured materials, our solutions help you achieve maximum density, finer microstructures, and enhanced mechanical properties—all while reducing processing time and temperature. Let us help you optimize your sintering process for breakthrough results. Contact our experts today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
People Also Ask
- Why is precise temperature control necessary for SiC/Cu Vacuum Hot Pressing? Mastering the Cu9Si Interface Phase
- What is the purpose of maintaining a vacuum environment in hot press sintering WCp/Cu? Ensure High Density & Purity
- What is vacuum hot pressing? Achieve Maximum Density & Purity in Advanced Materials
- What role does a vacuum hot pressing sintering furnace play in the fabrication of CuCrFeMnNi alloys? Achieve High Purity
- What is the purpose of using a hydraulic press to pre-press mixed powders? Optimize Your Sintering Success



















