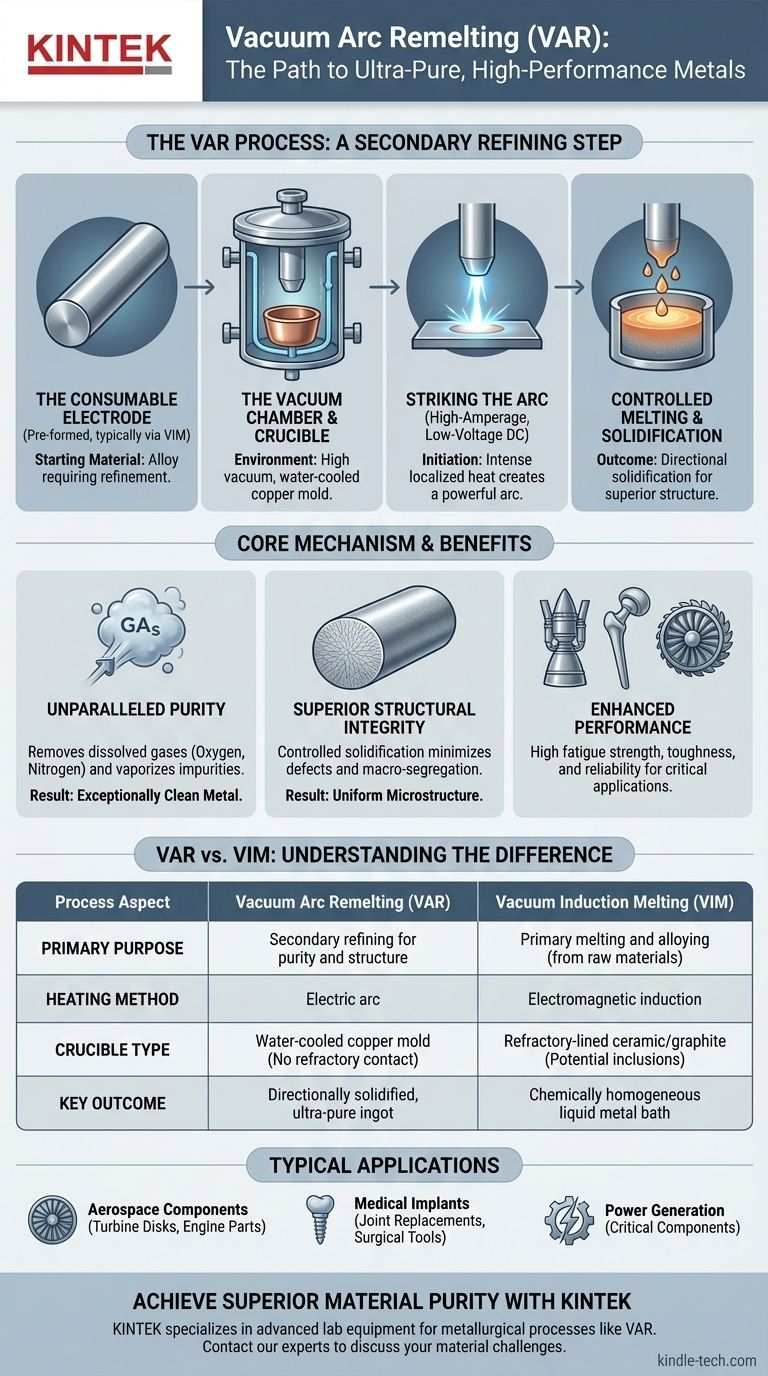
In short, vacuum consumable electrode melting, more commonly known as Vacuum Arc Remelting (VAR), is a secondary melting process used to purify and improve the quality of metals and superalloys. It works by using a high-current electric arc to progressively melt a solid metal electrode (the "consumable electrode") under a deep vacuum, allowing the molten metal to resolidify in a water-cooled copper mold, producing a highly pure and structurally uniform ingot.
The core purpose of Vacuum Arc Remelting is not to melt raw materials, but to refine an existing metal electrode, removing impurities and controlling the solidification process to create a final product with superior cleanliness, structural integrity, and mechanical properties.

The Core Mechanism of Vacuum Arc Remelting (VAR)
The VAR process is a highly controlled refining operation designed to produce the highest quality material possible. It is fundamentally different from primary melting methods like vacuum induction melting.
Step 1: The Consumable Electrode
The process begins with a pre-formed cylinder of the metal alloy that needs refining. This is the consumable electrode. It is typically created through a primary melting process like Vacuum Induction Melting (VIM).
Step 2: The Vacuum Chamber and Crucible
The electrode is suspended inside a sealed, water-cooled vacuum chamber. At the bottom of this chamber is a water-cooled copper crucible (or mold) that will contain the newly solidified ingot. The entire system is pumped down to a high vacuum.
Step 3: Striking the Arc
A high-amperage, low-voltage DC electrical potential is applied between the bottom of the electrode and a small amount of starter material in the base of the crucible. This initiates a powerful electric arc, which creates immense localized heat, similar to a welding arc.
Step 4: Controlled Melting and Solidification
The intense heat of the arc melts the tip of the consumable electrode. Metal droplets fall from the electrode into the shallow pool of molten metal in the copper crucible below.
Because the crucible is actively water-cooled, the molten metal solidifies progressively from the bottom up and from the outside in. This highly controlled cooling is critical for the final quality of the ingot.
Distinguishing VAR from Vacuum Induction Melting (VIM)
While both are vacuum processes, VAR and VIM serve different purposes and operate on different principles. The provided references describe VIM, so it is crucial to understand the distinction.
Heating Method and Purpose
VAR uses an electric arc to remelt an existing electrode for purification and structural refinement. It is a secondary, refining process.
VIM (Vacuum Induction Melting) uses electromagnetic induction to melt raw, solid metals (like scrap or elemental chunks) in a refractory-lined crucible. It is a primary melting and alloying process.
The "Crucible"
In VAR, the "crucible" is a water-cooled copper mold. Its purpose is to rapidly and directionally extract heat to control solidification. The molten metal never touches refractory materials, preventing contamination.
In VIM, the crucible is a ceramic or graphite vessel that must withstand high heat. This refractory lining can be a potential source of microscopic ceramic inclusions in the final melt.
Final Product Structure
VAR produces a directionally solidified ingot with a very fine, uniform grain structure and minimal chemical segregation.
VIM produces a chemically homogeneous liquid metal bath due to electromagnetic stirring, which is then cast into a mold. The solidification is less controlled than in VAR.
Understanding the Trade-offs: Why VAR is Used
VAR is an expensive and time-consuming process. It is reserved for applications where material failure is not an option.
Key Benefit: Unparalleled Purity
The combination of a high vacuum and high temperature removes dissolved gases like oxygen and nitrogen. It also vaporizes and extracts high-vapor-pressure tramp elements (impurities), resulting in an exceptionally clean metal.
Key Benefit: Superior Structural Integrity
The controlled, directional solidification minimizes defects like porosity and shrinkage cavities. It also produces a highly uniform microstructure, which is free of the macro-segregation (inconsistent alloy distribution) that can plague conventional castings.
Result: Enhanced Performance
This combination of purity and structural integrity gives VAR materials exceptional fatigue strength, toughness, and reliability. This is why the process is essential for producing materials used in aerospace turbine disks, medical implants, and power generation components.
Making the Right Choice for Your Goal
The selection of a melting process is dictated entirely by the cost and performance requirements of the final component.
- If your primary focus is creating a specific alloy from raw materials: VIM is the correct primary melting process for reactive alloys and superalloys.
- If your primary focus is achieving the absolute highest purity and structural integrity for a critical application: VAR is the necessary secondary refining step, almost always performed on an electrode previously made by VIM.
- If your primary focus is cost-effective production of less-critical components: Neither VIM nor VAR may be necessary, and simpler air-melting or electroslag remelting (ESR) processes might suffice.
Ultimately, understanding these advanced manufacturing processes empowers you to specify the precise material quality needed for your application's success.
Summary Table:
| Process Aspect | Vacuum Arc Remelting (VAR) | Vacuum Induction Melting (VIM) |
|---|---|---|
| Primary Purpose | Secondary refining for purity and structure | Primary melting and alloying |
| Heating Method | Electric arc | Electromagnetic induction |
| Crucible Type | Water-cooled copper mold | Refractory-lined ceramic/graphite |
| Key Outcome | Directionally solidified, ultra-pure ingot | Chemically homogeneous liquid metal |
| Typical Applications | Aerospace components, medical implants | Creating specific alloys from raw materials |
Need ultra-pure, high-performance metals for your critical applications? KINTEK specializes in advanced lab equipment and consumables for metallurgical processes like Vacuum Arc Remelting. Our expertise ensures you have the right tools to achieve the superior material purity and structural integrity required for aerospace, medical, and power generation components. Contact our experts today to discuss how we can support your laboratory's most demanding material challenges.
Visual Guide
Related Products
- Vacuum Arc Induction Melting Furnace
- Vacuum Induction Melting Spinning System Arc Melting Furnace
- Lab-Scale Vacuum Induction Melting Furnace
- Non Consumable Vacuum Arc Induction Melting Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the melting process in an induction furnace? A Guide to Clean, Efficient Metal Melting
- What is the power rating capacity for a medium frequency furnace? Find the Perfect kW for Your Melting Needs
- Why are ultra-high-temperature induction melting furnaces critical for Cr-based alloys? Mastering 2000°C+ Synthesis
- Can induction furnace melt iron? Unlock High-Efficiency, Clean Melting for Iron & Alloys
- What is melt loss? The Ultimate Guide to Reducing Metal Loss in High-Temp Processing
- What is the induction melting method? A Guide to Clean, Efficient Metal Melting
- Why do we use induction furnace? For Clean, Precise, and Efficient Metal Melting
- What is the primary advantage of the vacuum environment in this type of furnace? Achieve Oxidation-Free Precision



















