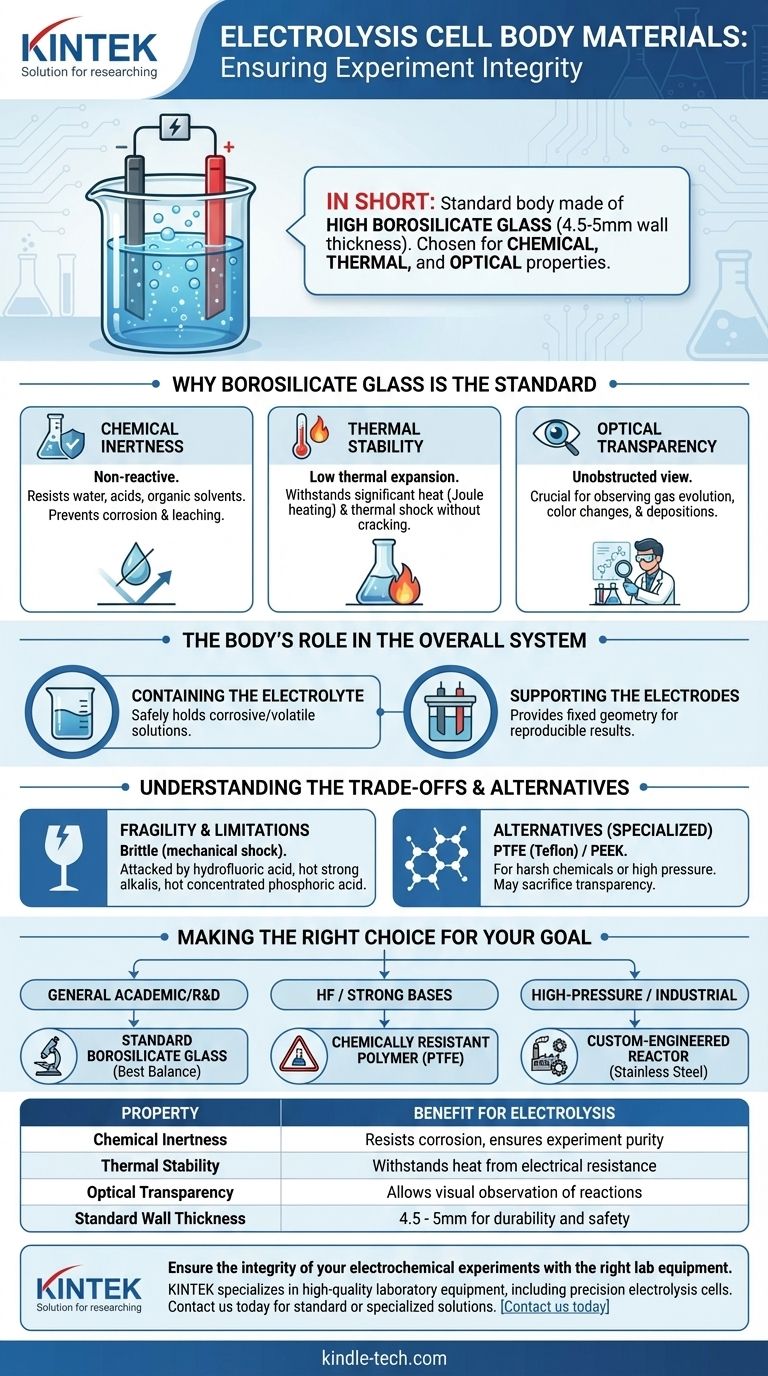
In short, the body of a standard electrolysis cell is typically constructed from high borosilicate glass. This specific type of glass, usually with a wall thickness between 4.5 and 5mm, is chosen for its unique combination of chemical, thermal, and optical properties that are essential for reliable electrochemical experiments.
The material for an electrolysis cell body is not just a container; it is a critical component engineered to be invisible to the chemical reaction it houses. The choice of high borosilicate glass ensures the experiment's integrity by providing chemical inertness, thermal stability, and full visibility.

Why Borosilicate Glass is the Standard
The selection of a material for an electrolysis cell is dictated by the harsh conditions within. The cell body must contain the electrolyte and electrodes without interfering with the sensitive electrochemical process.
Chemical Inertness
The primary requirement is that the cell body remains completely non-reactive. It must not corrode, leach impurities into the electrolyte, or react with the products being formed at the electrodes.
High borosilicate glass excels in this regard, showing exceptional resistance to water, acids, and a wide range of organic solvents. This ensures the purity of the experiment is maintained.
Thermal Stability
Electrolysis can generate significant heat (Joule heating) due to the electrical resistance of the electrolyte. The cell material must withstand these temperature changes without fracturing.
Borosilicate glass has a very low coefficient of thermal expansion, making it highly resistant to thermal shock. This allows it to handle the heat generated during an experiment without risk of cracking.
Optical Transparency
Visual observation is a critical part of many electrochemical experiments. Researchers need to see gas evolution on an electrode, color changes in the electrolyte, or the deposition of a metal.
The transparency of glass provides an unobstructed view of the cell's interior, which is a significant advantage over opaque materials like most plastics or metals.
The Body's Role in the Overall System
The cell body is more than a simple beaker. It is an integral part of an electrochemical system that includes the electrodes and the electrolyte.
Containing the Electrolyte
The most basic function is to safely hold the electrolyte, which is often a corrosive or volatile aqueous or organic solution that facilitates ion conduction between the electrodes.
Supporting the Electrodes
A well-designed cell body, often in conjunction with a lid, provides the necessary ports and structure to hold the working electrode (anode), counter electrode (cathode), and reference electrode in a fixed and stable geometry. This geometric consistency is crucial for reproducible results.
Understanding the Trade-offs
While borosilicate glass is the default choice, it is not without limitations. Understanding these trade-offs is key to selecting the right equipment.
The Fragility of Glass
The most obvious downside is that glass is brittle. It can chip or shatter if dropped or subjected to mechanical shock, which can lead to the loss of an experiment and a potential safety hazard.
Chemical Limitations
While highly resistant, borosilicate glass is not completely inert to all chemicals. It can be attacked by hydrofluoric acid, strong hot alkaline solutions, and hot concentrated phosphoric acid. In these specific cases, alternative materials are necessary.
Alternatives for Specialized Conditions
For applications involving chemicals that attack glass, or for high-pressure industrial systems, cells made from polymers like PTFE (Teflon) or PEEK are used. While these offer superior chemical resistance in some cases, they may sacrifice transparency or introduce other limitations.
Making the Right Choice for Your Goal
Your choice of cell material should be guided by the specific demands of your experiment or application.
- If your primary focus is general-purpose academic or R&D work: Standard borosilicate glass cells offer the best all-around balance of performance, visibility, and cost.
- If your primary focus is work with hydrofluoric acid or strong bases: A cell made from a chemically resistant polymer like PTFE is required.
- If your primary focus is high-pressure or industrial-scale electrolysis: You will likely need a custom-engineered reactor made from stainless steel or another robust material.
Ultimately, the cell material must be chosen to ensure it provides a stable and non-interfering environment for the reaction you wish to study.
Summary Table:
| Property | Benefit for Electrolysis |
|---|---|
| Chemical Inertness | Resists corrosion, ensures experiment purity |
| Thermal Stability | Withstands heat from electrical resistance |
| Optical Transparency | Allows visual observation of reactions |
| Standard Wall Thickness | 4.5 - 5mm for durability and safety |
Ensure the integrity of your electrochemical experiments with the right lab equipment. KINTEK specializes in high-quality laboratory equipment and consumables, including electrolysis cells designed for precision and reliability. Whether you need standard borosilicate glass cells or specialized alternatives for harsh chemicals, our experts can help you select the perfect solution for your laboratory's needs. Contact us today to discuss your requirements and enhance your research capabilities!
Visual Guide
Related Products
- Super Sealed Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should the H-type electrolytic cell be connected? Expert Setup Guide for Precise Electrochemical Experiments
- What is the overall structure of the H-type electrolytic cell? Understanding Dual-Chamber Electrochemical Designs
- What are the advantages of a PTFE-covered glass electrolytic cell? Ensure Precision in CO2-Saturated Testing
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- How does the design of an electrolytic cell influence evaluation of electrochemical catalytic performance? Key Factors



















