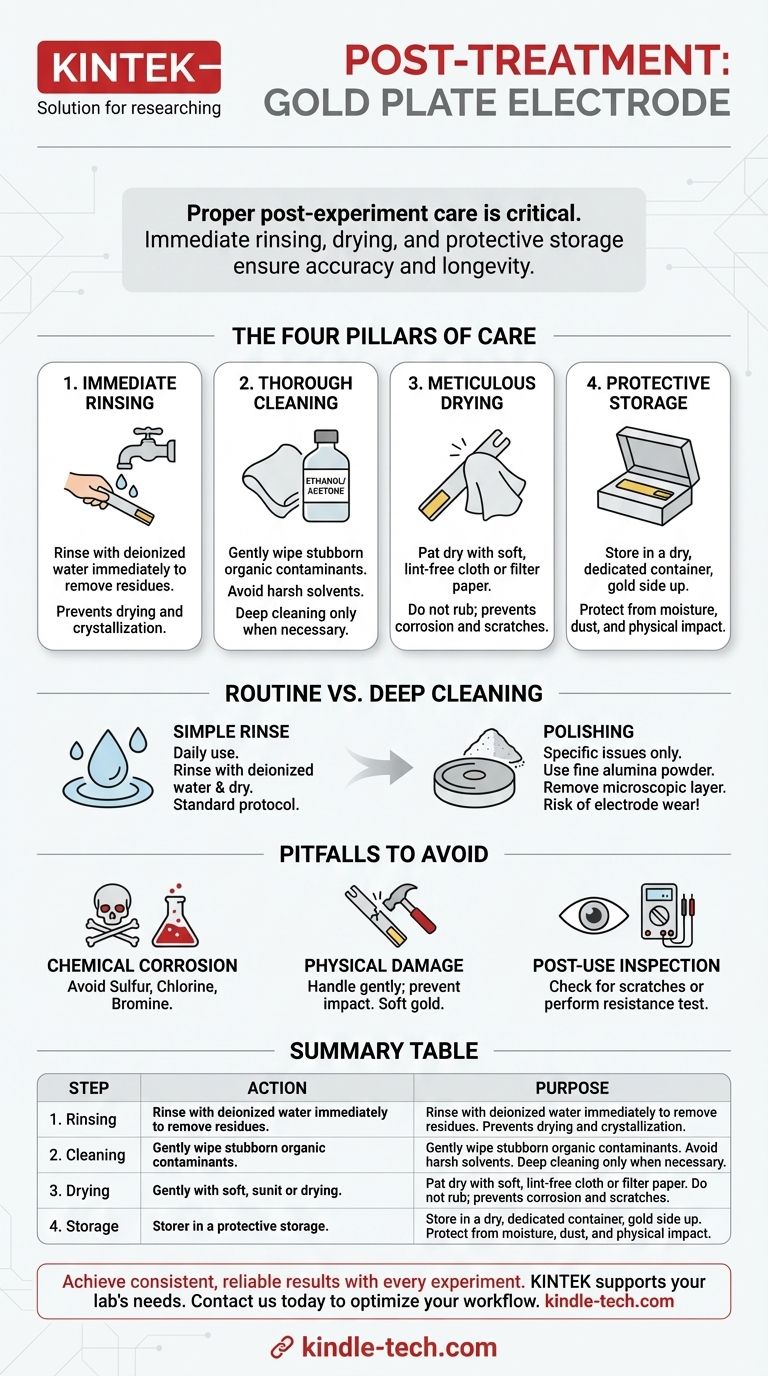
Proper post-experiment care is critical for maintaining the performance and extending the lifespan of a gold plate electrode. Immediately after use, you must dismantle the electrode, thoroughly rinse its surface with pure or deionized water to remove all residues, and gently dry it with a soft, non-abrasive material like a clean cloth or filter paper. Finally, store the clean, dry electrode in a dedicated container, with the gold surface facing upwards, in an environment free from moisture and contaminants.
The goal of post-treatment is not merely to clean the electrode, but to preserve the integrity of its sensitive surface. Following a strict protocol of immediate rinsing, proper drying, and protective storage is essential for ensuring the accuracy and reproducibility of your future experiments.

The Four Pillars of Electrode Post-Treatment
Properly caring for a gold electrode after an experiment is a systematic process. It can be broken down into four essential stages, each designed to protect the instrument from physical and chemical damage.
Pillar 1: Immediate Removal and Rinsing
The moment an experiment concludes, your first action should be to dismantle the electrode from the apparatus. This minimizes its exposure to potentially corrosive electrolytes or airborne contaminants.
Immediately rinse the electrode surface with deionized or pure water. This step is crucial for washing away residual salts and other substances before they can dry, crystallize, or react with the gold surface.
Pillar 2: Thorough and Appropriate Cleaning
For most routine experiments, a thorough rinse is sufficient. However, if you suspect stubborn residues have adhered to the surface, a deeper clean may be necessary.
Gently wipe the surface with a soft cloth moistened with ethanol or acetone to remove organic contaminants. Avoid harsh or unknown solvents that could damage the electrode.
If the surface is passivated or heavily contaminated, you may need to polish it. This is a more aggressive method that should be used sparingly.
Pillar 3: Meticulous Drying
After rinsing and cleaning, the electrode must be dried completely. Any residual moisture can promote corrosion or attract atmospheric contaminants during storage.
Use a soft, lint-free cloth or clean filter paper to gently pat the surface dry. Do not rub the surface, as the soft gold is susceptible to scratching.
Pillar 4: Protective Storage
Proper storage is the final and most critical step in preserving your electrode. The goal is to create a stable, inert environment.
Store the electrode in a dry, dedicated container, such as its original box. This protects it from physical impacts and dust.
Always place the electrode with the gold plate facing upwards to prevent the active surface from resting against any other material. For long-term storage, ensure it is protected from air, high temperatures, and strong light.
Understanding the Trade-offs: Routine vs. Deep Cleaning
Knowing when to perform a simple rinse versus a full polish is key to effective electrode maintenance. Using the wrong method can be either insufficient or damaging.
When a Simple Rinse Is Enough
For the vast majority of experiments, an immediate and thorough rinse with deionized water followed by careful drying is the correct procedure. This is the standard protocol for daily use.
When to Consider Polishing
Polishing should be reserved for specific situations, such as when you observe a visible film, experience a noticeable drop in performance, or are trying to restore an electrode with inconsistent results.
This process involves using a fine alumina powder (e.g., 0.05 µm) on a polishing pad to mechanically remove a microscopic layer from the electrode surface, revealing a fresh, clean layer of gold.
The Risks of Over-Polishing
Polishing is an abrasive process that physically removes gold from the electrode. Frequent or improper polishing will wear down the electrode, shorten its lifespan, and can alter its electrochemical properties over time.
Common Pitfalls to Avoid
Even with a proper procedure, simple mistakes can lead to irreversible damage. Awareness is your best defense.
Chemical Corrosion
Never expose the electrode to substances containing elements like sulfur, chlorine, or bromine, as they can aggressively corrode the gold surface. This includes storing the electrode in environments where these chemicals may be present in the air.
Physical Damage
The gold plate is extremely soft and prone to scratches, dents, and deformation. Always handle the electrode gently and prevent it from making contact with hard or sharp objects.
Post-Use Inspection
After cleaning, it is good practice to perform a quick inspection. Visually check for any new scratches or discoloration. If your equipment allows, a simple resistance test can help you identify developing connectivity or surface integrity issues before they compromise an experiment.
Making the Right Choice for Your Workflow
Your post-treatment protocol should align with your experimental needs and frequency of use.
- If your primary focus is routine daily use: Adhere strictly to the immediate rinse, dry, and protective storage cycle after every single experiment.
- If you are troubleshooting inconsistent results: Consider performing a controlled deep clean with a solvent, followed by polishing if performance does not improve.
- If you are preparing for long-term storage: Ensure the electrode is exceptionally clean and completely dry before sealing it in a dedicated, airtight container.
Consistent, careful maintenance is the foundation of reliable electrochemical data.
Summary Table:
| Post-Treatment Step | Key Action | Purpose |
|---|---|---|
| 1. Immediate Rinsing | Rinse with deionized water immediately after use. | Remove residual salts/electrolytes before they dry. |
| 2. Thorough Cleaning | Gently wipe with ethanol/acetone for stubborn residues. | Eliminate organic contaminants without damaging the surface. |
| 3. Meticulous Drying | Pat dry with a soft, lint-free cloth or filter paper. | Prevent corrosion and contamination from moisture. |
| 4. Protective Storage | Store in a dry container with the gold surface facing up. | Shield from physical damage, dust, and airborne contaminants. |
Achieve consistent, reliable results with every experiment. Proper electrode maintenance is fundamental to research integrity. KINTEK specializes in high-precision lab equipment and consumables, including electrodes and cleaning supplies, to support your laboratory's needs. Let our experts help you optimize your workflow and protect your investment. Contact us today to discuss your specific requirements and ensure your equipment performs at its best.
Visual Guide
Related Products
- Gold Disc Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Proton Exchange Membrane for Batteries Lab Applications
People Also Ask
- How should a gold disc electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis



















