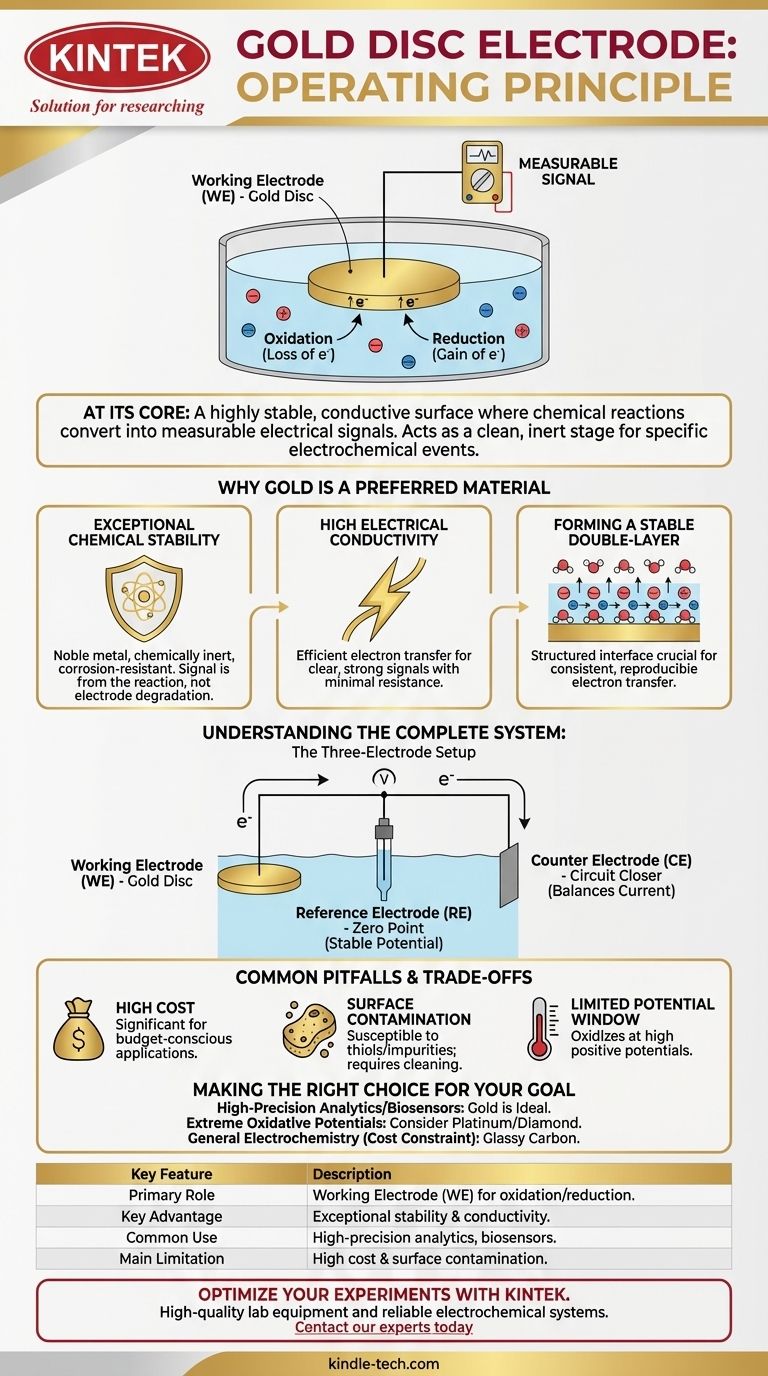
At its core, a gold disc electrode operates as a highly stable and conductive surface where chemical reactions are converted into measurable electrical signals. In an electrochemical system, it typically functions as the working electrode, the specific site where electrons are exchanged with ions in a solution, driving the oxidation or reduction reaction you intend to study.
The fundamental principle of a gold disc electrode is not merely to conduct electricity, but to act as a clean, inert stage for a specific electrochemical event. Its value comes from its ability to facilitate this electron transfer predictably, allowing the resulting current or potential to be a direct measure of the chemical reaction itself.

The Role of the Working Electrode
The Site of the Main Event
In a typical three-electrode setup, the gold disc is the Working Electrode (WE). This is the centerpiece of your experiment—the location where the primary electrochemical reaction of interest takes place.
Converting Chemistry to Electricity
When the electrode is submerged in an electrolyte solution and a potential is applied, it creates an electric field at its surface. This field forces chemical species in the solution to either give up electrons to the electrode (oxidation) or take electrons from it (reduction).
A Gateway for Electron Flow
This transfer of electrons generates an electrical current that flows through an external circuit. By measuring this current or the potential at which it occurs, we can gain insight into the reaction's kinetics, concentration of reactants, and underlying mechanisms.
Why Gold is a Preferred Material
Exceptional Chemical Stability
Gold is a noble metal, meaning it is chemically inert and highly resistant to corrosion or oxidation under most conditions. This ensures that the measured current is from your reaction of interest, not from the electrode itself degrading.
High Electrical Conductivity
Gold's excellent conductivity allows for efficient and rapid transfer of electrons between the chemical species and the external measurement circuit. This results in a clear, strong signal with minimal electrical resistance.
Forming a Stable Double-Layer
At the interface between the gold surface and the electrolyte solution, a structured layer of ions and solvent molecules forms, known as the electrochemical double-layer. Gold's stable surface allows this layer to form predictably, which is crucial for consistent and reproducible electron transfer.
Understanding the Complete System
The Three-Electrode Setup
The working electrode does not operate in a vacuum. To get meaningful data, it is part of a system that includes two other electrodes.
The Reference Electrode (The Zero Point)
This electrode provides a stable, constant potential that acts as a reliable reference point. The potential of the gold working electrode is measured against this stable benchmark, much like measuring the height of a mountain relative to sea level. Crucially, almost no current flows through it, preserving its stability.
The Counter Electrode (The Circuit Closer)
The counter electrode (or auxiliary electrode) serves to complete the electrical circuit. It passes all the current that flows through the working electrode, ensuring that the reference electrode remains undisturbed and the overall system is balanced.
Common Pitfalls and Trade-offs
High Cost
The most obvious drawback of gold is its high price compared to other electrode materials like glassy carbon. This can be a significant factor in budget-conscious applications.
Surface Contamination
Gold surfaces are notoriously susceptible to contamination. They readily bind with sulfur-containing compounds (thiols) and other impurities, which can block the active surface and alter experimental results if not cleaned properly.
Limited Potential Window
While chemically stable, gold itself can begin to oxidize at high positive potentials. This limits the range of reactions that can be studied without the electrode itself interfering with the measurement.
Making the Right Choice for Your Goal
- If your primary focus is high-precision analytics: Gold's stability and predictable surface make it an ideal choice for developing sensitive and reproducible sensors.
- If you are studying biological molecules or building biosensors: Gold is an excellent platform, as molecules can be easily and stably attached (immobilized) to its surface.
- If your experiment involves extreme oxidative potentials: You may need to consider more robust materials like platinum or boron-doped diamond to avoid interference from gold oxidation.
- If cost is a primary constraint for general electrochemistry: A glassy carbon electrode often provides a suitable and much more affordable alternative for a wide range of applications.
Ultimately, understanding the gold electrode's role as an active and stable interface is the key to designing and interpreting any electrochemical experiment.
Summary Table:
| Key Feature | Description |
|---|---|
| Primary Role | Acts as the Working Electrode (WE) for oxidation/reduction reactions. |
| Key Advantage | Exceptional chemical stability and high electrical conductivity. |
| Common Use | Ideal for high-precision analytics, biosensors, and biological molecule studies. |
| Main Limitation | High cost and susceptibility to surface contamination. |
Optimize your electrochemical experiments with the right equipment. KINTEK specializes in high-quality lab equipment and consumables, including reliable electrodes and electrochemical systems. Our expertise ensures you get the precision and stability needed for reproducible results. Contact our experts today to discuss how we can support your laboratory's specific needs!
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
People Also Ask
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis
- What is the proper post-treatment and storage procedure for a gold disc electrode? Ensure Reliable Electrochemical Data
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data



















