In a typical electrochemical setup, a gold disc electrode serves as the working electrode (WE). This means it is the primary site of interest where the electrochemical reaction—either oxidation or reduction—is controlled and measured.
To study an electrochemical reaction, you require a stable, controlled surface where electron transfer can be precisely monitored. The gold disc electrode provides an inert, highly conductive, and well-defined area for this purpose, making it the central component where the chemistry of your experiment unfolds.

The Role of the Working Electrode
The working electrode is the centerpiece of most electrochemical experiments. Its function is to act as the surface where the specific reaction you want to investigate takes place.
The Site of the Reaction
All measurements are centered on the working electrode's surface. It is at this interface between the solid gold and the liquid electrolyte solution that electrons are either supplied to a chemical species (reduction) or removed from it (oxidation).
The WE can function as either the anode (where oxidation occurs) or the cathode (where reduction occurs), depending on the potential applied to it by the experimental equipment (a potentiostat).
The "Material of Interest"
The working electrode is the "stage" for your experiment. The results you measure—the current—are a direct consequence of the chemical events happening on its surface.
In some cases, you study the properties of the gold itself. More often, you are studying a substance dissolved in the solution, and the gold electrode simply acts as an inert surface to facilitate the electron transfer.
Why Use Gold Specifically?
While many conductive materials can be used, gold is a popular choice for several key reasons that make it ideal for sensitive and reproducible measurements.
Chemical Stability
Gold is a noble metal, meaning it is chemically stable and relatively inert. It resists oxidation and corrosion in many common electrolyte solutions.
This inertness ensures that the current you measure is from your reaction of interest, not from an unintended reaction involving the electrode material itself.
Excellent Conductivity
Gold has outstanding electrical conductivity. This property allows for efficient and rapid transfer of electrons between the electrode and the species in the solution, which is critical for accurate measurements.
Well-Defined Surface Chemistry
A gold surface can be cleaned and prepared to be highly uniform and reproducible. It is also well-known for its ability to bind specific molecules, particularly those containing sulfur, making it a cornerstone of biosensor development and surface science.
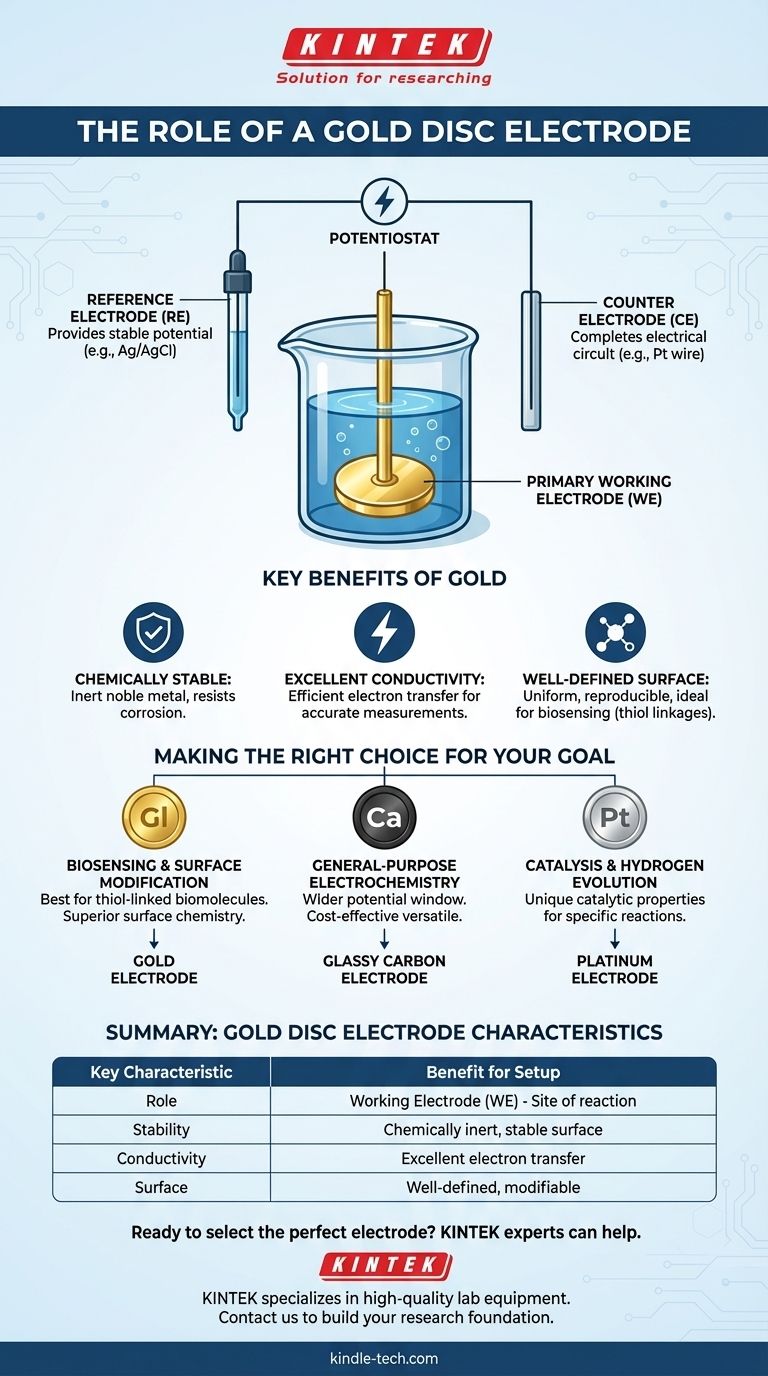
Understanding the Full Three-Electrode System
A gold disc electrode does not work in isolation. It is one part of a standard three-electrode system, which is necessary for making accurate electrochemical measurements.
The Working Electrode (WE)
As discussed, this is the electrode where the reaction of interest occurs. Its potential is controlled, and the resulting current is measured.
The Reference Electrode (RE)
The reference electrode provides a stable, constant potential that does not change during the experiment. Think of it as the "sea level" of voltage; the potential of the working electrode is measured against this stable reference point.
The Counter Electrode (CE)
The counter electrode (or auxiliary electrode) completes the electrical circuit. It passes all the current needed by the working electrode, ensuring that no significant current flows through the sensitive reference electrode, which would destabilize its potential.
Understanding the Trade-offs and Alternatives
While powerful, a gold electrode is not always the best choice for every application. Understanding its limitations is key to good experimental design.
The Cost of Gold
Gold is an expensive material. For routine or large-scale experiments, the cost can be a significant factor compared to other materials.
Limited Potential Window
In certain solutions, particularly acidic or chloride-containing ones, gold can begin to oxidize at high positive potentials. This "anodic limit" restricts the range of experiments that can be performed.
Common Alternatives: Platinum and Carbon
Platinum (Pt) is another noble metal frequently used as a working electrode, especially in studies involving catalysis (like hydrogen evolution or oxygen reduction).
Glassy carbon (GC) is a very common and more affordable alternative. It is highly inert, has a very wide potential window in both positive and negative directions, and is suitable for a broad range of general-purpose electrochemical analyses.
Making the Right Choice for Your Goal
Selecting the correct working electrode material is fundamental to the success of your experiment. Your choice depends directly on your analytical objective.
- If your primary focus is biosensing or surface modification: Gold is often the superior choice due to its well-understood surface chemistry for immobilizing biomolecules via thiol linkages.
- If your primary focus is general-purpose electrochemistry: A glassy carbon electrode is often a more versatile and cost-effective starting point due to its wider potential window.
- If your primary focus is catalysis or specific reactions like hydrogen evolution: A platinum electrode is typically the standard material due to its unique catalytic properties.
Ultimately, selecting the right working electrode is the first step toward designing a precise and meaningful electrochemical experiment.
Summary Table:
| Key Characteristic | Benefit for Electrochemical Setup |
|---|---|
| Role | Serves as the Working Electrode (WE) |
| Primary Function | Site for controlled oxidation/reduction reactions |
| Material Stability | Chemically inert, resists corrosion |
| Conductivity | Excellent for efficient electron transfer |
| Surface Chemistry | Well-defined, ideal for biosensing and modification |
| Common Alternative | Glassy Carbon (wider potential window) |
Ready to select the perfect electrode for your experiment?
KINTEK specializes in high-quality lab equipment and consumables, including a range of working electrodes for precise electrochemical analysis. Whether your focus is biosensing, catalysis, or general-purpose electrochemistry, our experts can help you choose the right tool for your laboratory's needs.
Contact our team today to discuss your application and ensure your research is built on a solid foundation.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
People Also Ask
- What is the proper post-treatment and storage procedure for a gold disc electrode? Ensure Reliable Electrochemical Data
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data



















