To ensure accurate results and a long lifespan for your gold disc electrode, you must adhere to a strict protocol covering physical handling, chemical exposure, electrical parameters, and cleaning. The core precautions involve protecting the soft gold surface from physical damage, avoiding corrosive chemicals like sulfur and halogens, operating within its specified voltage limits, and performing meticulous polishing and cleaning before and after each use.
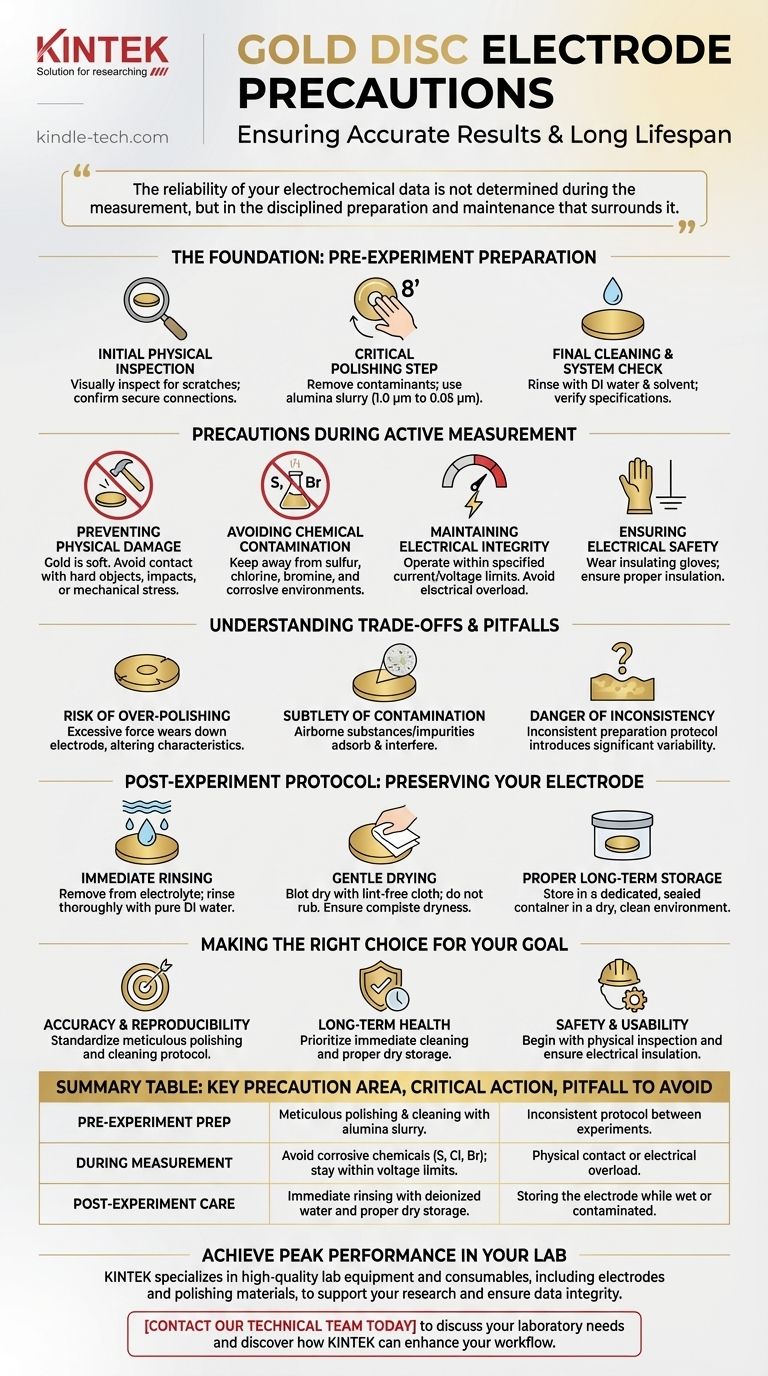
The reliability of your electrochemical data is not determined during the measurement, but in the disciplined preparation and maintenance that surrounds it. A pristine, reproducible electrode surface is the prerequisite for any successful experiment.

The Foundation: Pre-Experiment Preparation
Before any measurement can be taken, the electrode surface must be brought to a known, clean, and active state. This preparation phase is the most critical step for achieving reproducible results.
The Initial Physical Inspection
First, visually inspect the electrode. Look for any obvious scratches, dents, or physical deformation on the gold surface.
Also, confirm that the connecting wire and housing are secure, as a loose connection can introduce significant noise into your measurements.
The Critical Polishing Step
The goal of polishing is to remove any adsorbed contaminants or oxide layers and create a fresh, uniform surface.
Place a small amount of polishing powder, such as alumina (starting with 1.0 µm and finishing with 0.05 µm), onto a polishing cloth moistened with distilled water.
Hold the electrode vertically and polish the surface using a gentle figure '8' or circular motion. This ensures even abrasion across the disc.
Final Cleaning and System Check
After polishing, rinse the electrode thoroughly with deionized water, followed by a solvent like ethanol or acetone to remove any residual polishing slurry.
Gently dry the surface. Before immersing it, ensure the electrolyte is of the correct purity and concentration as required by your experiment.
Finally, verify that the electrode's specifications are compatible with your potentiostat and cell setup.
Precautions During Active Measurement
Once the experiment is running, your focus shifts to protecting the electrode from its operational environment.
Preventing Physical Damage
The gold surface is extremely soft and can be easily damaged. Avoid any contact with hard objects, impacts, or mechanical stress, which can alter the electrode's surface area and compromise your results.
Avoiding Chemical Contamination
Keep the electrode away from solutions or environments containing elements known to corrode or passivate gold.
Key substances to avoid include sulfur, chlorine, and bromine, which can irreversibly alter the surface chemistry.
Maintaining Electrical Integrity
Always operate the electrode within its specified current and voltage tolerance range. Exceeding these limits can cause irreversible damage to the electrode surface or the internal connection.
Ensuring Electrical Safety
Wear insulating gloves during operation. Ensure any metal stands or clamps that could contact the electrolyte or power sources are properly insulated to prevent electric shock.
Understanding the Trade-offs and Pitfalls
Proper electrode care requires balancing competing factors and being aware of subtle sources of error.
The Risk of Over-Polishing
While polishing is essential, it is also an abrasive process. Over-polishing or using excessive force can gradually wear down the electrode, changing its dimensions and electrochemical characteristics over time.
The Subtlety of Contamination
Contamination is not always visible. Airborne substances, residue from cleaning agents, or impurities in your electrolyte can adsorb onto the gold surface and interfere with sensitive measurements.
The Danger of Inconsistency
The single largest source of non-reproducible results is an inconsistent preparation protocol. Using different polishing times, pressures, or cleaning steps between experiments will introduce significant variability.
Post-Experiment Protocol: Preserving Your Electrode
What you do immediately after an experiment is critical for the long-term health and performance of the electrode.
Immediate Rinsing
As soon as the experiment is complete, remove the electrode from the electrolyte. Rinse it thoroughly with pure, deionized water to remove any residual salts or reactants.
Gentle Drying
Carefully blot the electrode surface dry using filter paper or a soft, lint-free cloth. Do not wipe or rub the surface. Ensure the electrode is completely dry before storage to prevent corrosion.
Proper Long-Term Storage
Store the clean, dry electrode in a dedicated, sealed container away from dust and corrosive fumes. It should be kept in a dry environment, protected from high temperatures and strong light.
Making the Right Choice for Your Goal
Your primary objective dictates which precautions are most critical in your workflow.
- If your primary focus is obtaining highly accurate and reproducible data: Your most critical task is to standardize a meticulous pre-experiment polishing and cleaning protocol and execute it identically every time.
- If your primary focus is ensuring the long-term health of the electrode: Your priority is immediate post-experiment cleaning and proper dry storage to prevent surface degradation over time.
- If your primary focus is safety and general usability: Always begin with a physical inspection of the electrode and its wiring, and ensure all electrical components are properly insulated before starting your experiment.
A well-maintained electrode is not just a tool; it is the foundation of reliable electrochemical data.
Summary Table:
| Key Precaution Area | Critical Action | Pitfall to Avoid |
|---|---|---|
| Pre-Experiment Prep | Meticulous polishing & cleaning with alumina slurry. | Inconsistent protocol between experiments. |
| During Measurement | Avoid corrosive chemicals (S, Cl, Br); stay within voltage limits. | Physical contact or electrical overload. |
| Post-Experiment Care | Immediate rinsing with deionized water and proper dry storage. | Storing the electrode while wet or contaminated. |
Achieve Peak Performance and Reproducibility in Your Lab
Your electrochemical experiments demand precision. KINTEK specializes in high-quality lab equipment and consumables, including electrodes and polishing materials, to support your research and ensure data integrity. Let our experts help you select the right tools and establish best practices for your specific application.
Contact our technical team today to discuss your laboratory needs and discover how KINTEK can enhance your workflow.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Metal Disc Electrode Electrochemical Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- What is the operating principle of a gold disc electrode in an electrochemical system? Unlock Precision with a Stable Interface
- What is the material and purity of a gold disc electrode? Ensuring Precision in Electrochemical Analysis
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data



















