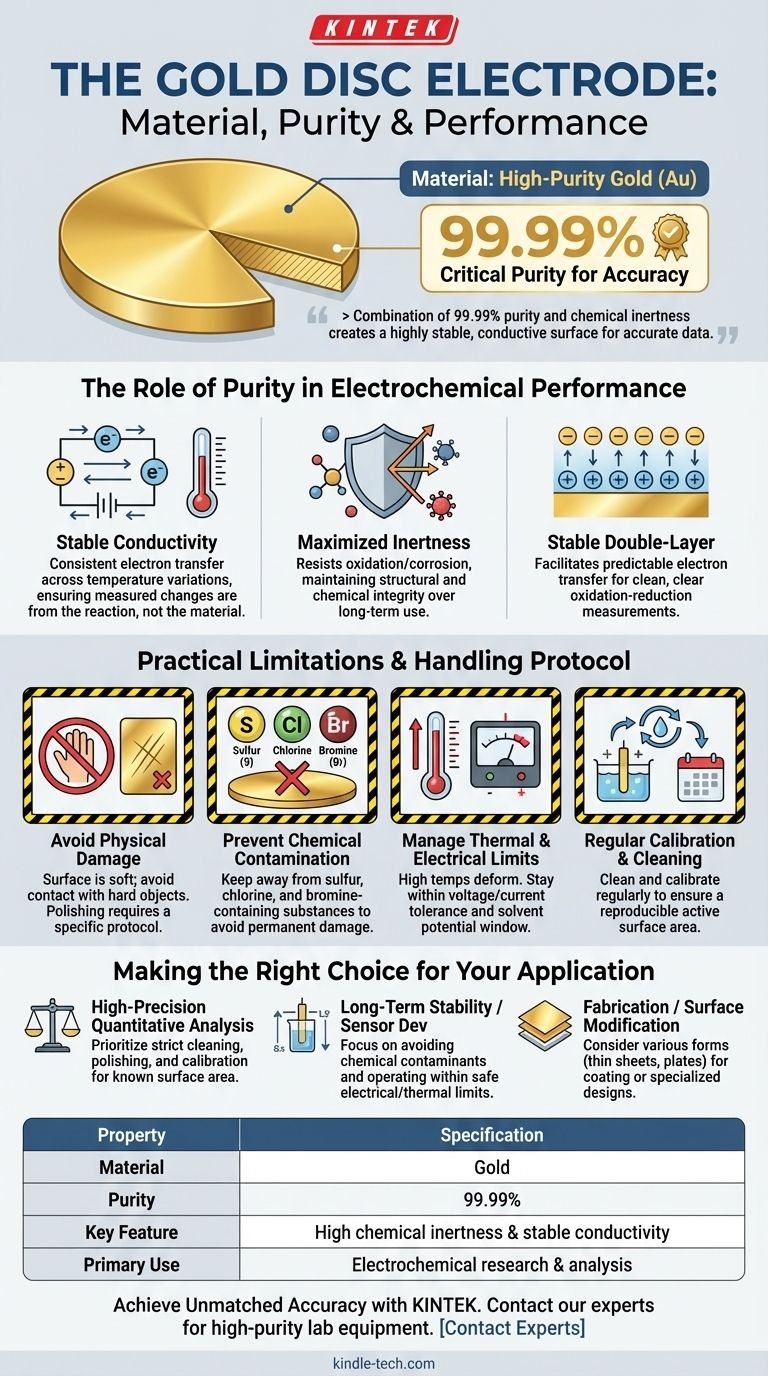
At its core, a gold disc electrode is made of high-purity gold, typically rated at 99.99%. This specific purity level is not arbitrary; it is a critical requirement for ensuring the electrode performs with the high degree of accuracy and reliability demanded in electrochemical research and analysis. The material's inherent properties are leveraged to create a stable and predictable interface for studying chemical reactions.
A gold disc electrode's value comes from its combination of 99.99% purity and gold's natural chemical inertness. This pairing creates a highly stable and conductive surface, which is essential for obtaining accurate and reproducible electrochemical data.

The Role of Purity in Electrochemical Performance
The 99.99% purity standard directly contributes to the electrode's function as a reliable analytical tool. Impurities, even in small amounts, could introduce unpredictable side reactions or alter the electrode's conductive properties, compromising experimental results.
Ensuring Electrical Conductivity and Stability
A key property of high-purity gold is its exceptionally stable electrical conductivity. This means its ability to conduct electrons remains consistent across variations in temperature and other environmental conditions.
This stability ensures that any measured changes in current are due to the electrochemical reaction being studied, not fluctuations in the electrode material itself.
Maximizing Chemical Inertness
Gold is a noble metal, known for its excellent corrosion resistance and chemical stability. The 99.99% purity minimizes the presence of other, more reactive elements on the electrode's surface.
This prevents the electrode from being easily oxidized or corroded by the electrolyte solution, ensuring it maintains its structural and chemical integrity over long-term use.
Forming a Stable Double-Layer
In an electrolyte, the electrode surface and the ions in the solution form an electrical double-layer. The stability and predictability of this layer are crucial for facilitating electron transfer.
Because of its chemical inertness and conductivity, high-purity gold forms a very stable and well-characterized double-layer, allowing for clean and clear measurement of oxidation-reduction reactions.
Practical Limitations and Handling Protocol
While robust, a gold electrode's performance is dependent on proper handling. Its physical and chemical properties define its operational limits.
Avoiding Physical Damage
The surface of a gold electrode is relatively soft and can be easily scratched or deformed. Contact with hard objects must be avoided, as surface imperfections can alter its effective surface area and electrochemical behavior.
For precise measurements, the surface is often polished to a mirror finish using a specific protocol, a process that must be done with care.
Preventing Chemical Contamination
Despite its general inertness, gold can be corroded by specific elements. It is critical to keep the electrode away from substances containing sulfur, chlorine, and bromine.
Exposure to these elements can permanently damage the electrode surface and render it unsuitable for sensitive measurements.
Managing Thermal and Electrical Limits
Excessively high temperatures can cause the electrode to deform. Likewise, the applied voltage and current must be kept within the electrode's tolerance range and the potential window of the solvent.
Exceeding these limits can lead to irreversible damage to the electrode or cause unintended reactions that interfere with the experiment.
The Need for Regular Calibration and Cleaning
For any application requiring precise data, the electrode must be regularly cleaned and often calibrated. The active surface area can change over time due to adsorption of species from the solution.
A consistent cleaning protocol, often involving electrochemical cycles or polishing, is necessary to ensure reproducible results between experiments.
Making the Right Choice for Your Application
Understanding these material properties is key to using a gold electrode effectively. Your experimental goal will dictate your primary focus.
- If your primary focus is high-precision quantitative analysis: You must prioritize a strict cleaning, polishing, and calibration protocol to ensure a known and reproducible surface area.
- If your primary focus is long-term stability or sensor development: You must pay close attention to avoiding chemical contaminants (like sulfur or chlorides) and operating within safe electrical and thermal limits.
- If your primary focus is fabrication or surface modification: Be aware that gold is available in various forms, such as thin sheets or plates, which may be more suitable for coating or specialized cell designs than a traditional disc electrode.
Ultimately, treating your gold electrode as the precise instrument it is will ensure the integrity of your electrochemical data.
Summary Table:
| Property | Specification |
|---|---|
| Material | Gold |
| Purity | 99.99% |
| Key Feature | High chemical inertness & stable conductivity |
| Primary Use | Electrochemical research & analysis |
Achieve Unmatched Accuracy in Your Lab
For precise electrochemical measurements, the integrity of your equipment is paramount. KINTEK specializes in high-purity lab equipment and consumables, including gold disc electrodes, to ensure your research is built on a foundation of reliability and precision.
Contact our experts today to discuss how our solutions can enhance your laboratory's performance and data quality.
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
People Also Ask
- How should a gold disc electrode be maintained for long-term use? A Guide to Consistent Performance
- What is the typical role of a gold disc electrode in an electrochemical setup? Your Guide to a Precise Working Electrode
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data
- How should a gold disc electrode be handled during an experiment? Ensure Accurate Electrochemical Measurements
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan



















