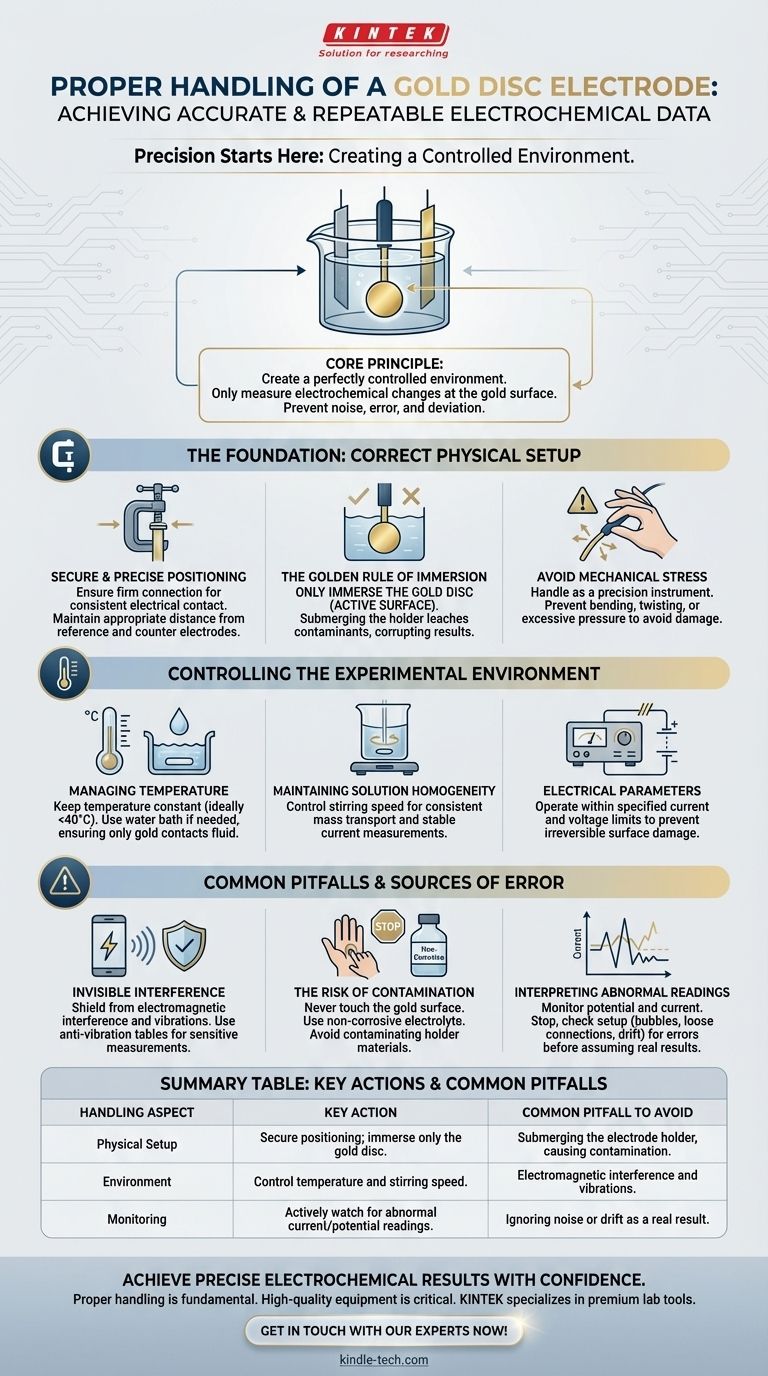
Proper handling of a gold disc electrode during an experiment is critical for acquiring accurate and repeatable data. It requires correct physical installation within the electrochemical cell, strict control over experimental conditions like temperature and stirring, and active monitoring of the electrode's performance while shielding it from external interference.
The core principle is to create a perfectly controlled environment where the only changes measured are from the electrochemical reaction at the gold surface. Any deviation in setup, environment, or external influence introduces noise and error, fundamentally undermining the validity of your results.
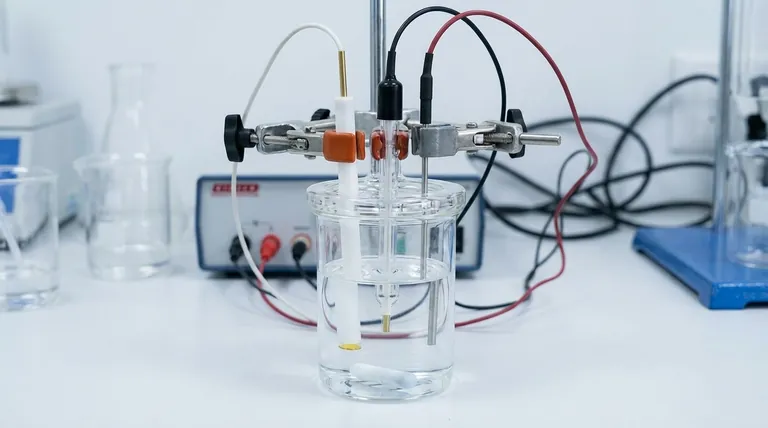
The Foundation: Correct Physical Setup
The physical arrangement of your electrochemical cell is the first and most critical step. Errors made here will cascade through your entire measurement.
Secure and Precise Positioning
Your gold working electrode must be installed securely in the apparatus. A firm connection prevents poor electrical contact, which is a common source of erratic data.
The electrode's position relative to the reference and counter electrodes is also crucial. Maintain an appropriate and consistent distance to ensure a uniform electric field and accurate potential measurement.
The Golden Rule of Immersion
Only the active surface—the gold disc itself—should be immersed in the electrolyte solution.
Submerging the electrode holder or clip is a frequent and serious mistake. The sealants and materials in the holder can degrade upon prolonged contact with the electrolyte, leaching contaminants into your solution and corrupting your experiment.
Avoid Mechanical Stress
The electrode assembly is a precision instrument. Avoid subjecting it to any bending, twisting, impact, or excessive pressure during installation or adjustment. Such stress can damage the internal connections or the seal between the electrode body and the gold surface.
Controlling the Experimental Environment
Your experiment does not exist in a vacuum. The surrounding environment must be managed just as carefully as the electrode itself.
Managing Temperature
Electrochemical reaction rates are highly sensitive to temperature. Unless temperature is the variable you are studying, it must be kept constant.
It is generally recommended to conduct experiments at a stable ambient temperature, ideally not exceeding 40°C, to protect the electrode's construction materials. If temperature control is needed, a water bath can be used, but again, ensure only the gold portion of the electrode contacts the fluid.
Maintaining Solution Homogeneity
For many experiments, a controlled stirring speed is required to ensure a consistent mass transport of reactants to the electrode surface. An unstable or incorrect stirring rate will cause fluctuations in your measured current.
Electrical Parameters
Always operate within the electrode's specified current and voltage limits. Exceeding these parameters can cause irreversible damage to the electrode surface or induce unwanted side reactions.
Common Pitfalls and Sources of Error
Even with a perfect setup, external factors can ruin an experiment. Awareness is the best defense.
"Invisible" Interference
Electrochemical measurements involve very small electrical signals that are easily disrupted. Keep your setup away from sources of electromagnetic interference, such as power supplies, motors, or mobile phones.
Mechanical vibrations can also introduce noise by altering the diffusion layer at the electrode surface. Consider using an anti-vibration table for highly sensitive measurements.
The Risk of Contamination
Contamination is the enemy of electrochemistry. Besides the risk from the electrode holder, ensure your chosen electrolyte is non-corrosive and does not react with the electrode materials. Never touch the gold surface with your bare hands.
Interpreting Abnormal Readings
During the experiment, closely monitor the potential and current. If you observe sudden spikes, drifts, or excessive noise, it often indicates a problem.
Stop the experiment and systematically check for issues: Has the reference electrode drifted? Is there a bubble on the electrode surface? Has a connection come loose? Do not assume the strange data is a real result without first ruling out experimental error.
Applying This to Your Experiment
Use these principles to guide your actions based on your specific experimental goals.
- If your primary focus is high-precision quantitative analysis: You must prioritize absolute stability. Use shielding for electromagnetic interference, a water bath for temperature control, and a vibration-dampening table.
- If your primary focus is material characterization or screening: Your priority is preventing major errors. Double-check that only the gold disc is immersed and ensure your connections are secure.
- If you are troubleshooting inconsistent results: Systematically review every point in this guide. Start with the physical setup, especially immersion depth and the stability of your reference electrode, as these are the most common sources of error.
Mastering the handling of your electrode transforms it from a source of frustration into a reliable tool for discovery.
Summary Table:
| Handling Aspect | Key Action | Common Pitfall to Avoid |
|---|---|---|
| Physical Setup | Secure positioning; immerse only the gold disc. | Submerging the electrode holder, causing contamination. |
| Environment | Control temperature and stirring speed. | Electromagnetic interference and vibrations. |
| Monitoring | Actively watch for abnormal current/potential readings. | Ignoring noise or drift as a real result. |
Achieve precise and reliable electrochemical results with confidence. Proper electrode handling is fundamental, but having the right, high-quality equipment is just as critical. KINTEK specializes in premium lab equipment and consumables, providing the reliable tools your laboratory needs for success. Contact us today to discuss how our solutions can support your specific experimental requirements and enhance your data quality.
Get in touch with our experts now!
Visual Guide
Related Products
- Gold Disc Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Glassy Carbon Electrochemical Electrode
People Also Ask
- What is the typical role of a gold disc electrode in an electrochemical setup? Your Guide to a Precise Working Electrode
- What are the key precautions for a gold disc electrode? Ensure Accurate Results & Long Lifespan
- What are the necessary pretreatment steps before using a gold disc electrode? A Guide to Reliable Electrochemical Data
- What are the key aspects of maintaining and caring for a gold plate electrode? Preserve Performance and Extend Lifespan
- What are the performance characteristics of a gold plate electrode? Unmatched Stability for Reliable Data

















