When cleaning an electrolytic cell, the two most critical practices to avoid are using abrasive tools like metal brushes and mixing acidic and alkaline cleaning agents. These actions can cause irreversible physical damage to the cell's surfaces and create a significant chemical safety hazard from a violent exothermic reaction.
The core principle of cleaning an electrolytic cell is preservation. Your goal is not just to remove contaminants but to do so while maintaining the physical integrity of the cell and ensuring your own safety, which requires a methodical and gentle approach.

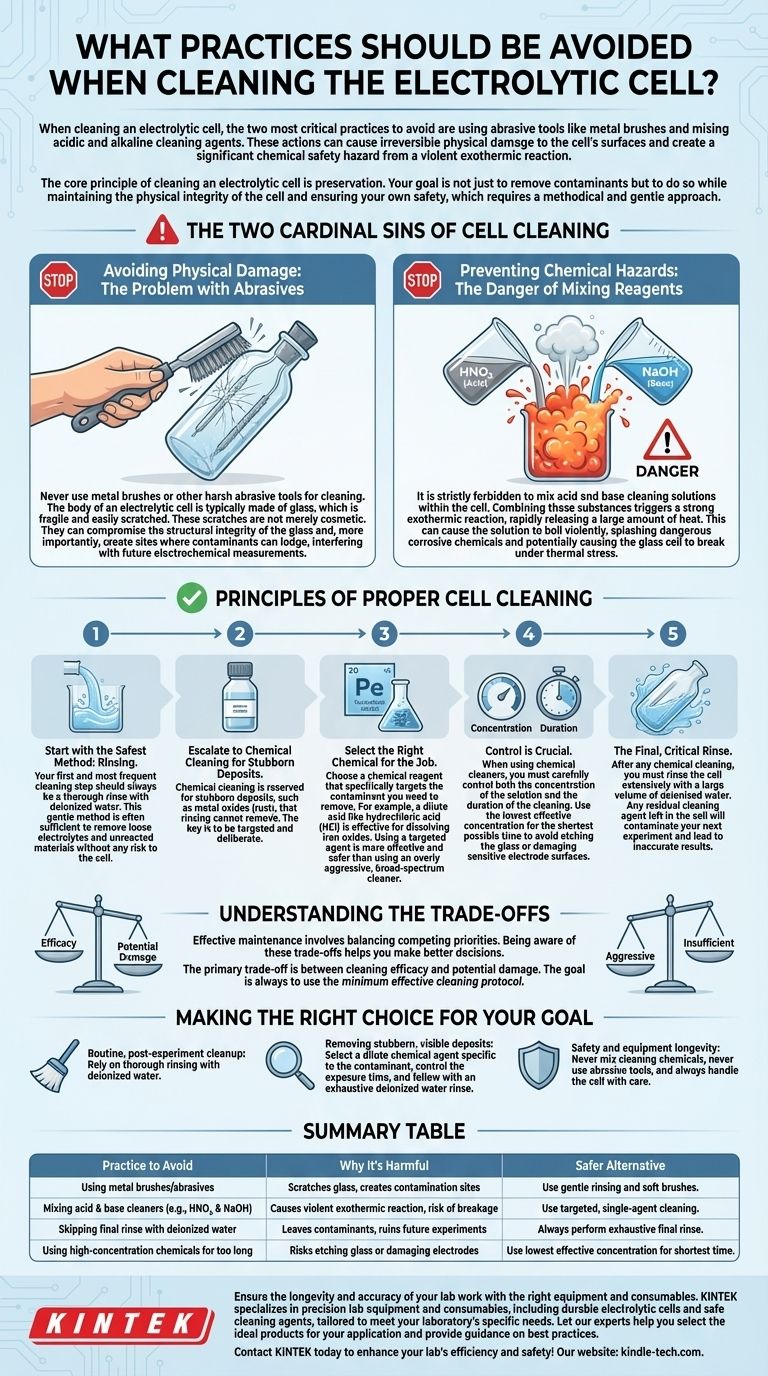
The Two Cardinal Sins of Cell Cleaning
To ensure the longevity and accuracy of your electrolytic cell, you must be aware of two fundamental prohibitions that prevent the most common and severe forms of damage.
Avoiding Physical Damage: The Problem with Abrasives
Never use metal brushes or other harsh abrasive tools for cleaning. The body of an electrolytic cell is typically made of glass, which is fragile and easily scratched.
These scratches are not merely cosmetic. They can compromise the structural integrity of the glass and, more importantly, create sites where contaminants can lodge, interfering with future electrochemical measurements.
Preventing Chemical Hazards: The Danger of Mixing Reagents
It is strictly forbidden to mix acid and base cleaning solutions, such as nitric acid (HNO₃) and sodium hydroxide (NaOH), within the cell.
Combining these substances triggers a strong exothermic reaction, rapidly releasing a large amount of heat. This can cause the solution to boil violently, splashing dangerous corrosive chemicals and potentially causing the glass cell to break under thermal stress.
Principles of Proper Cell Cleaning
Avoiding bad practices is only half the battle. Understanding the correct, methodical approach is essential for effective and safe maintenance.
Start with the Safest Method: Rinsing
Your first and most frequent cleaning step should always be a thorough rinse with deionized water. This gentle method is often sufficient to remove loose electrolytes and unreacted materials without any risk to the cell.
Escalate to Chemical Cleaning for Stubborn Deposits
Chemical cleaning is reserved for stubborn deposits, such as metal oxides (rust), that rinsing cannot remove. The key is to be targeted and deliberate.
Select the Right Chemical for the Job
Choose a chemical reagent that specifically targets the contaminant you need to remove. For example, a dilute acid like hydrochloric acid (HCl) is effective for dissolving iron oxides. Using a targeted agent is more effective and safer than using an overly aggressive, broad-spectrum cleaner.
Control is Crucial
When using chemical cleaners, you must carefully control both the concentration of the solution and the duration of the cleaning. Use the lowest effective concentration for the shortest possible time to avoid etching the glass or damaging sensitive electrode surfaces.
The Final, Critical Rinse
After any chemical cleaning, you must rinse the cell extensively with a large volume of deionized water. Any residual cleaning agent left in the cell will contaminate your next experiment and lead to inaccurate results.
Understanding the Trade-offs
Effective maintenance involves balancing competing priorities. Being aware of these trade-offs helps you make better decisions.
Aggressive vs. Insufficient Cleaning
The primary trade-off is between cleaning efficacy and potential damage. Over-aggressive cleaning with strong chemicals or physical scrubbing will shorten the cell's lifespan, while insufficient cleaning will leave behind contaminants that ruin your data. The goal is always to use the minimum effective cleaning protocol.
Forgetting Personal Safety
Handling chemical cleaning agents requires diligence. Always wear appropriate Personal Protective Equipment (PPE), including gloves and safety goggles. Perform chemical cleaning in a well-ventilated area or a fume hood to avoid inhaling hazardous vapors.
Ignoring the Cell's Fragility
Remember that the cell is a piece of precision lab equipment. It must be handled gently at all times, not just during the cleaning process itself. A moment of carelessness can lead to breakage.
Making the Right Choice for Your Goal
Apply these principles based on your specific cleaning needs to ensure the best outcome for your equipment and your experiments.
- If your primary focus is routine, post-experiment cleanup: Rely on thorough rinsing with deionized water as your default method.
- If your primary focus is removing stubborn, visible deposits: Select a dilute chemical agent specific to the contaminant, control the exposure time, and follow with an exhaustive deionized water rinse.
- If your primary focus is safety and equipment longevity: Never mix cleaning chemicals, never use abrasive tools, and always handle the cell with care.
Proper cleaning is a critical part of good science, ensuring both the integrity of your data and the longevity of your valuable equipment.
Summary Table:
| Practice to Avoid | Why It's Harmful | Safer Alternative |
|---|---|---|
| Using metal brushes/abrasives | Scratches glass, creates contamination sites | Use gentle rinsing and soft brushes |
| Mixing acid & base cleaners (e.g., HNO₃ & NaOH) | Causes violent exothermic reaction, risk of breakage | Use targeted, single-agent cleaning |
| Skipping final rinse with deionized water | Leaves contaminants, ruins future experiments | Always perform exhaustive final rinse |
| Using high-concentration chemicals for too long | Risks etching glass or damaging electrodes | Use lowest effective concentration for shortest time |
Ensure the longevity and accuracy of your lab work with the right equipment and consumables. Proper maintenance starts with quality labware. KINTEK specializes in precision lab equipment and consumables, including durable electrolytic cells and safe cleaning agents, tailored to meet your laboratory's specific needs.
Let our experts help you select the ideal products for your application and provide guidance on best practices. Contact KINTEK today to enhance your lab's efficiency and safety!
Visual Guide
Related Products
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What are the key features of the five-port water bath electrolytic cell? Precision Control for Electrochemical Experiments
- How can the electrochemical reaction be controlled when using this electrolytic cell? Master Voltage, Current & Electrolyte
- What are the standard aperture sizes on the lid of the multifunctional electrolytic cell? Key Ports for Your Electrochemical Setup



















