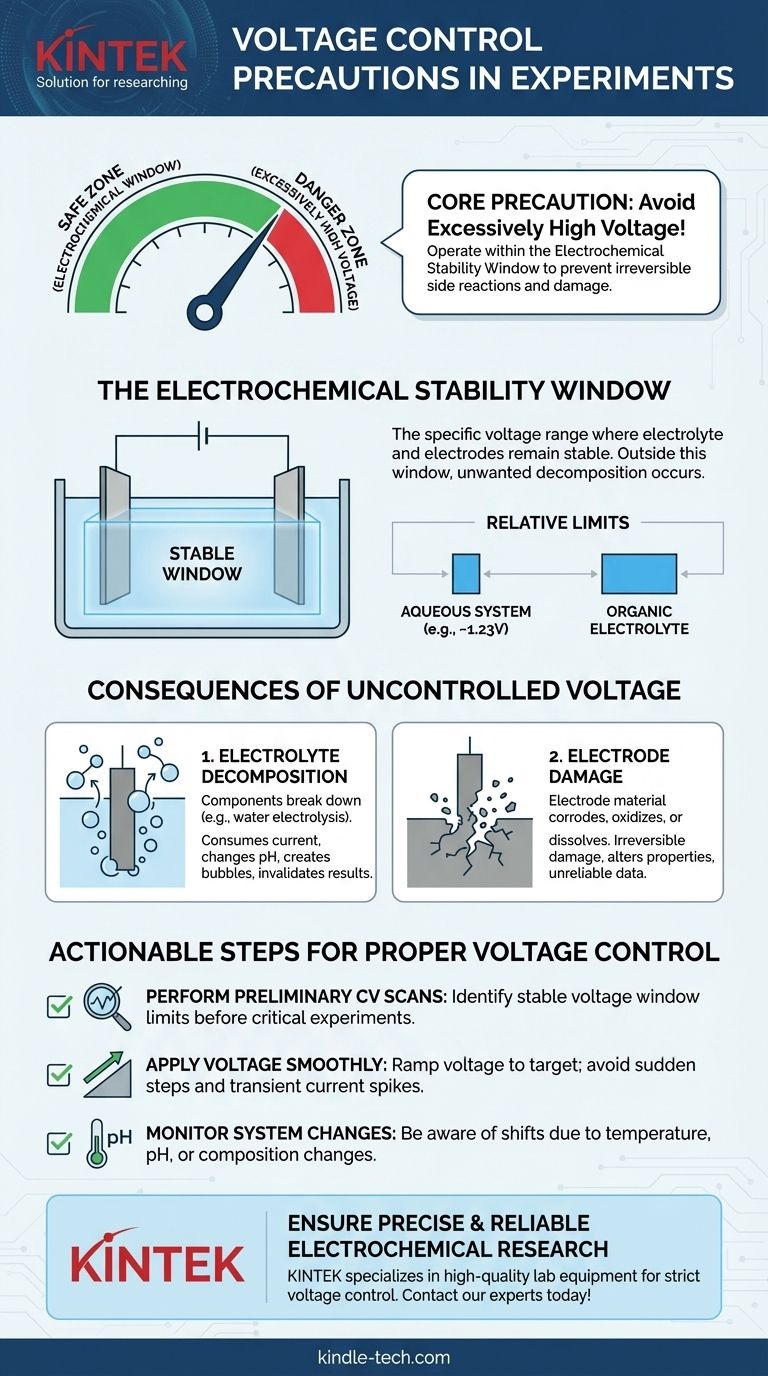
The single most critical precaution for voltage control in an electrochemical experiment is to avoid applying excessively high voltage. Applying a potential that is too great for your specific setup will compromise the integrity of your entire experiment by causing irreversible and unintended side reactions.
Your primary goal in voltage control is to operate within the electrochemical stability window of your system. Exceeding this voltage range does not simply accelerate your desired reaction; it introduces new, unwanted reactions that invalidate your results and can permanently damage your equipment.

The Core Problem: Exceeding the Electrochemical Window
To control voltage effectively, you must first understand the limits of the materials you are working with. The concept of an electrochemical window defines these limits.
What is the Electrochemical Window?
The electrochemical window is the specific voltage range where your electrolyte and electrodes remain stable and do not react. Within this window, the measurements you take are related to the process you intend to study.
Outside this window, the voltage is high enough to drive decomposition reactions of the solvent, the solute, or the electrode itself.
Why "Excessively High" is Relative
A voltage that is perfectly safe for one system can be destructive to another. The stable window is determined by the specific combination of your electrode material and your electrolyte solution.
For example, the stability window for an aqueous (water-based) electrolyte is fundamentally limited by the voltage at which water splits into hydrogen and oxygen. In contrast, certain organic electrolytes and ionic liquids offer much wider stability windows, allowing for experiments at higher potentials.
The Consequences of Uncontrolled Voltage
Applying a voltage beyond the stable window leads to two primary failure modes, both of which will corrupt your experimental data.
Consequence 1: Electrolyte Decomposition
This is often the first process to occur when the voltage is too high. The components of your electrolyte solution begin to break down chemically.
In an aqueous solution, this means the electrolysis of water, producing hydrogen and oxygen gas. This unwanted reaction consumes current, changes the local pH near the electrodes, and can create bubbles that block the electrode surface, invalidating your results.
Consequence 2: Electrode Damage
Excessive voltage can also directly damage the electrodes. High potentials can cause the electrode material to corrode, oxidize, or dissolve into the electrolyte.
This damage is often irreversible. It permanently alters the electrode's surface area and catalytic properties, making any subsequent measurements unreliable and inconsistent with previous tests.
Common Pitfalls to Avoid
Maintaining proper voltage control means being proactive and understanding your system's characteristics before running a critical experiment.
Ignoring Preliminary Scans
Before running a lengthy experiment, it is crucial to determine the electrochemical window of your specific setup. A quick cyclic voltammetry (CV) scan over a wide potential range will reveal the onset potentials for electrolyte or electrode decomposition.
Sudden Voltage Application
Always ramp the voltage to your target potential smoothly rather than applying it instantaneously. Sudden voltage steps can create large transient currents that may overshoot the stable window, causing momentary damage that can still affect your results.
Neglecting System Changes
The stability window can change if the electrolyte's composition, pH, or temperature changes. Be aware that what was a safe voltage at the beginning of an experiment may become excessive if the conditions drift over time.
How to Ensure Proper Voltage Control
Your approach to setting voltage limits should be directly tied to your experimental goals and materials.
- If you are working with a new system: Always perform a preliminary cyclic voltammetry (CV) scan to experimentally identify the stable voltage window before proceeding with other measurements.
- If you are performing a standard aqueous experiment: Be mindful of the theoretical water decomposition potential (~1.23 V) and set your voltage limits well within the range where water is stable.
- If your primary focus is long-term stability or precision: Operate comfortably in the middle of the established stability window, avoiding the edges where minor, slow decomposition reactions might begin to occur.
Ultimately, disciplined voltage control is the bedrock of valid, repeatable, and reliable electrochemical research.
Summary Table:
| Precaution | Purpose | Consequence of Neglect |
|---|---|---|
| Operate within the electrochemical window | Maintain system stability | Unwanted side reactions, invalid data |
| Perform preliminary CV scans | Identify safe voltage limits | Electrolyte decomposition (e.g., water electrolysis) |
| Apply voltage smoothly (ramp) | Avoid transient current spikes | Electrode corrosion or dissolution |
| Monitor for system changes (pH, temperature) | Ensure consistent stability window | Drifting conditions leading to voltage exceedance |
Ensure your electrochemical experiments are precise and reliable. Uncontrolled voltage can lead to irreversible damage and invalid results. KINTEK specializes in high-quality lab equipment and consumables for electrochemical research, helping you maintain strict voltage control and achieve accurate, repeatable data. Contact our experts today to discuss your specific laboratory needs and find the right solutions for your research.
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Platinum Auxiliary Electrode for Laboratory Use
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Gold Disc Electrode
People Also Ask
- What optical features does the H-type electrolytic cell have? Precision Quartz Windows for Photoelectrochemistry
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What are the key features of a double-layer water-bath electrolytic cell? Achieve Precise Temperature Control for Your Experiments



















