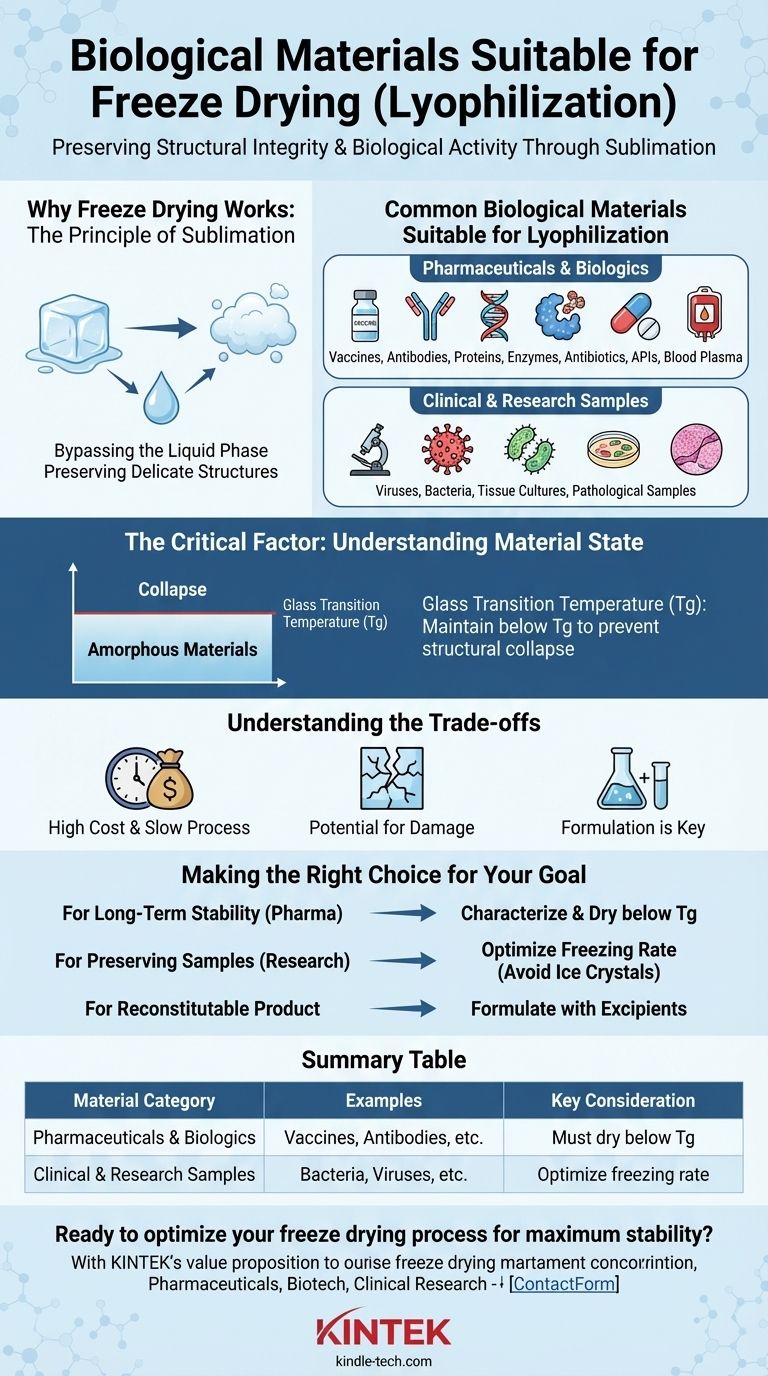
A wide range of biological materials are suitable for freeze drying, a process technically known as lyophilization. This includes sensitive products like vaccines, antibodies, proteins, and enzymes, as well as clinical samples such as blood plasma, bacteria, and tissue cultures. The key to successful preservation lies not just in the material itself, but in controlling the process to maintain the material's structural integrity at a molecular level.
The suitability of a biological material for freeze drying depends less on what it is and more on its ability to form a stable, solid, "glass-like" state. The goal is to keep the material below its critical temperature during the drying process to prevent structural collapse and preserve its activity.

Why Freeze Drying Works: The Principle of Sublimation
Freeze drying is the gold standard for preserving delicate biological materials because it minimizes the damage caused by water.
Bypassing the Liquid Phase
The process involves first freezing the material, then placing it under a deep vacuum. This allows the frozen water to turn directly from a solid (ice) into a gas (water vapor) in a process called sublimation.
By completely bypassing the liquid phase, sublimation avoids the destructive forces of surface tension that can denature proteins and rupture cells.
Preserving Delicate Structures
Without liquid water, the complex three-dimensional structures of proteins, enzymes, and other macromolecules are locked in place. This preserves their biological activity, making the process ideal for sensitive pharmaceuticals and research materials.
Common Biological Materials Suitable for Lyophilization
While the list is extensive, suitable materials generally fall into two major categories based on their application.
Pharmaceuticals and Biologics
This is the most common application, where stability and long shelf life are paramount.
- Vaccines and Antibodies: Lyophilization creates a stable, easily transportable powder that can be reconstituted before injection.
- Proteins, Peptides, and Enzymes: These large, fragile molecules are preserved with their activity intact.
- Antibiotics and APIs: Many active pharmaceutical ingredients (APIs) are stabilized for storage and transport.
- Blood Plasma: Removing water from plasma allows for long-term storage without refrigeration.
Clinical and Research Samples
Freeze drying is also used to stabilize specimens for later analysis or use.
- Viruses and Bacteria: Cultures are preserved in a state of suspended animation for research or probiotic use.
- Pathological Samples: Tissues and other pathological specimens are dried for histological or analytical studies without structural distortion.
The Critical Factor: Understanding Material State
The success of freeze drying hinges on understanding the physical state of the frozen material. Most biological formulations are not simple crystalline structures.
Amorphous Materials
Most biological products are amorphous, meaning they are multi-component mixtures that don't form a neat crystal structure when frozen. Instead, they solidify into a viscous, glass-like state.
The Importance of the Glass Transition Temperature (Tg)
For these amorphous materials, the most critical parameter is the glass transition temperature (Tg). This is the temperature below which the material is in a solid, stable, "glassy" state.
If the temperature during the primary drying phase rises above the Tg, the material softens and flows, leading to a loss of structure known as collapse. This ruins the product, compromising its appearance, stability, and biological activity. Therefore, the entire sublimation process must occur below this critical temperature.
Understanding the Trade-offs
While powerful, lyophilization is not a perfect or universal solution. It involves clear trade-offs that must be considered.
High Cost and Slow Process
Freeze dryers are expensive capital equipment, and the process itself is very slow, often taking several days. This makes it one of the most energy-intensive and time-consuming preservation methods.
Potential for Damage
If the process is not carefully optimized for the specific product, damage can still occur. Freezing rates that are too slow can create large ice crystals that damage cell structures, while improper vacuum or temperature control can lead to product collapse.
Formulation is Key
Many materials cannot be freeze-dried effectively on their own. They require the addition of excipients (stabilizing agents), such as sugars or polymers, to raise the glass transition temperature and protect the molecules during drying and storage.
Making the Right Choice for Your Goal
Your approach to freeze drying should be dictated by your end goal for the material.
- If your primary focus is long-term stability for pharmaceuticals: You must precisely characterize your formulation's glass transition temperature (Tg) and perform drying well below it.
- If your primary focus is preserving tissues or cultures for analysis: Optimizing the initial freezing rate is critical to prevent large ice crystals from forming and physically damaging the sample's structure.
- If your primary focus is creating a reconstitutable product: Formulating with the right excipients is essential to ensure the final dried "cake" is stable and dissolves easily.
Ultimately, successful freeze drying is a science of control, ensuring your material remains in a stable solid state from beginning to end.
Summary Table:
| Material Category | Examples | Key Consideration |
|---|---|---|
| Pharmaceuticals & Biologics | Vaccines, Antibodies, Proteins, Enzymes, Blood Plasma | Must dry below glass transition temperature (Tg) to prevent collapse |
| Clinical & Research Samples | Bacteria, Viruses, Tissue Cultures, Pathological Specimens | Optimize freezing rate to prevent ice crystal damage |
Ready to optimize your freeze drying process for maximum stability?
At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored for precise lyophilization. Whether you're preserving sensitive pharmaceuticals, clinical samples, or research materials, our solutions ensure you maintain structural integrity and biological activity.
We help you:
- Select the right equipment for your specific biological materials.
- Understand critical parameters like glass transition temperature (Tg).
- Achieve consistent, reliable results with long-term stability.
Targeting laboratories in pharmaceuticals, biotech, and clinical research, KINTEK is your partner for all freeze drying needs. Contact us today to discuss how we can support your preservation goals!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Small Injection Molding Machine for Lab Use
- Laboratory Hybrid Tissue Grinding Mill
People Also Ask
- Why is freeze drying considered more effective than ordinary drying? Preserve Structure, Nutrients & Flavor
- How are lab freeze dryers utilized in pharmaceutical research and development? Stabilize Drug Candidates with Lyophilization
- What role does freeze drying play in scientific research? Preserve Sample Integrity for Reliable Results
- What are the advantages of using a laboratory freeze dryer? Achieve Unmatched Sample Preservation
- Where are evaporators used in food industry? Concentrate Products & Reduce Costs



















