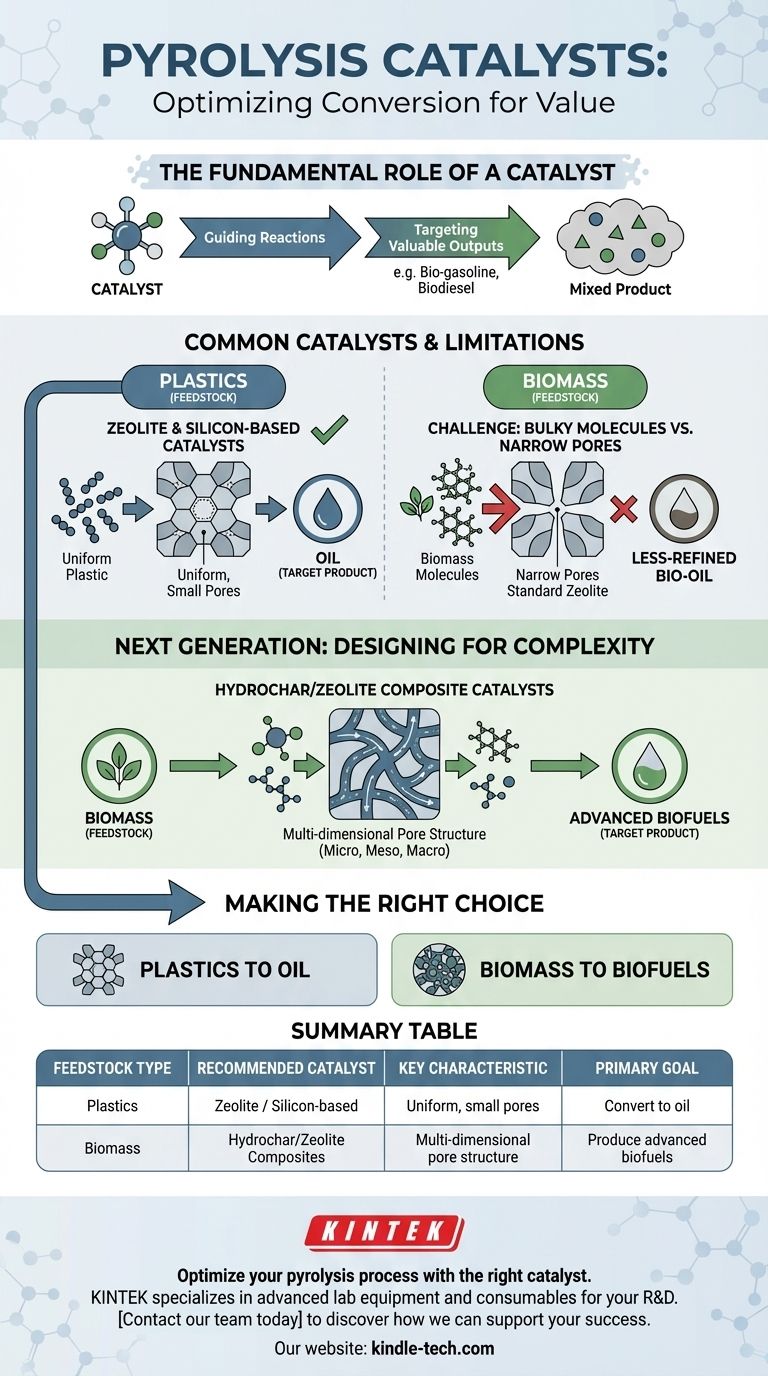
In pyrolysis, the most common catalysts are silicon and zeolite-based commercial catalysts, which are primarily used for converting materials like plastic into oil. However, the specific catalyst choice is highly dependent on the feedstock being processed, with newer composite catalysts being developed for more complex materials like biomass.
The core challenge in pyrolysis is not just finding a catalyst, but finding the right catalyst. The physical structure of the material being broken down—from simple plastics to bulky biomass—dictates the necessary structure of the catalyst itself.

The Fundamental Role of a Catalyst in Pyrolysis
A catalyst is introduced into the pyrolysis process to control the chemical reactions that occur as a material is heated in the absence of oxygen. Its primary job is to steer the conversion process toward specific, desirable products.
Guiding Chemical Reactions
Without a catalyst, pyrolysis can produce a wide mix of products, including liquid hydrocarbons (bio-oil), solid char, and various gases. A catalyst provides a surface where specific reactions can happen more efficiently.
Targeting Valuable Outputs
By promoting certain reactions, a catalyst can increase the yield and quality of a target product, such as transportation-grade fuels like bio-gasoline or biodiesel, instead of a less-refined crude bio-oil.
Common Catalysts and Their Limitations
While several catalysts are used, the most established options face significant challenges, particularly when moving beyond petrochemicals like plastic.
Zeolite and Silicon-Based Catalysts
For converting plastics, zeolite and silicon-based catalysts are the commercial standard. Their internal structure and pore size are well-suited for the relatively small and uniform molecules found in petrochemicals.
The Challenge with Biomass
These standard catalysts often fail when used for biomass pyrolysis. Natural polymers in biomass are much larger and bulkier than petrochemical molecules.
The narrow pores of conventional zeolites effectively block these larger molecules, preventing them from accessing the active sites where the catalytic conversion happens. This severely limits their effectiveness for producing advanced biofuels from biomass.
The Next Generation: Designing for Complexity
To overcome the limitations of traditional catalysts, research is focused on creating new structures specifically designed for the challenges of biomass.
Creating Multi-Dimensional Pores
The key innovation is developing catalysts with a multidimensional pore structure. This means creating a network of micro, meso, and macro-sized pores.
This hierarchical structure acts as a "highway system" for molecules. Larger pores allow bulky biomass derivatives to enter the catalyst, while smaller pores handle the subsequent reactions, dramatically improving molecular traffic control.
Hydrochar/Zeolite Composites
A promising example of this new approach is the hydrochar/zeolite composite catalyst. This design facilitates the diffusion of large molecules deep inside the catalyst.
By doing so, it vastly increases the number of accessible active sites, making the entire process more efficient and suitable for producing high-value biofuels from complex biomass.
Making the Right Choice for Your Goal
Selecting the correct catalyst is not a one-size-fits-all decision; it is dictated entirely by your feedstock and your desired end product.
- If your primary focus is converting plastics into oil: Standard commercial catalysts, such as zeolites, are a well-established and effective choice.
- If your primary focus is converting bulky biomass into advanced biofuels: You must look to next-generation, multi-porous composite catalysts like hydrochar/zeolite to overcome the physical limitations of the feedstock.
Ultimately, the future of efficient pyrolysis conversion lies in tailoring the catalyst's architecture to the unique nature of the material it is meant to transform.
Summary Table:
| Feedstock Type | Recommended Catalyst | Key Characteristic | Primary Goal |
|---|---|---|---|
| Plastics | Zeolite / Silicon-based | Uniform, small pores | Convert to oil |
| Biomass | Hydrochar/Zeolite Composites | Multi-dimensional pore structure | Produce advanced biofuels |
Optimize your pyrolysis process with the right catalyst. The efficiency of your conversion from plastic or biomass to valuable fuels depends heavily on selecting a catalyst tailored to your specific feedstock. KINTEK specializes in providing advanced lab equipment and consumables for pyrolysis research and development. Our experts can help you identify the ideal catalytic solutions to maximize your yield and product quality. Contact our team today to discuss your project needs and discover how KINTEK can support your laboratory's success.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Custom PTFE Teflon Parts Manufacturer for Hollow Etching Flower Basket ITO FTO Developing Glue Removal
- Advanced Engineering Fine Ceramics Alumina Ceramic Saggar for Fine Corundum
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are the advantages of using PTFE jars for RuTi alloy mixing? Ensure Chemical Purity and High Yield
- Why is it necessary to utilize PTFE sample holders in electroless nickel plating? Ensure Process Integrity
- What are the advantages of using PTFE molds for epoxy resin flame retardant samples? Ensure High-Purity Material Testing
- How are PTFE gaskets utilized for POEGMA electrolyte conductivity? Ensure Precision in Electrochemical Measurements
- Why are Polytetrafluoroethylene (PTFE) molds preferred for UV-cured siloxane films? Ensure Damage-Free Sample Release



















