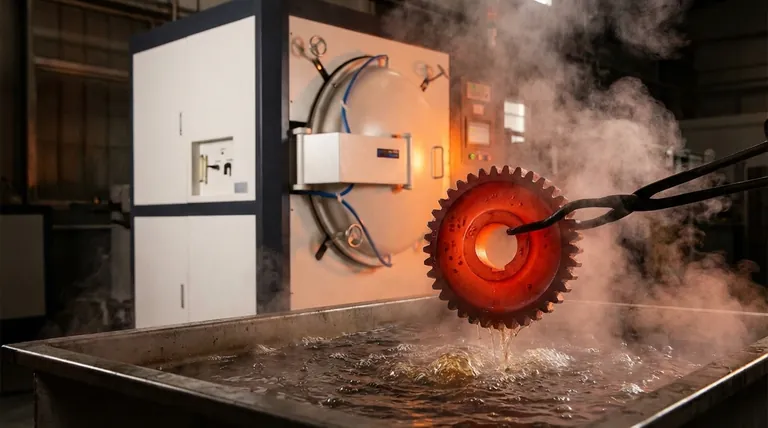
For achieving the absolute highest hardness in steel, the process is known as quenching. This involves heating the steel to a high temperature to transform its structure into austenite and then cooling it with extreme rapidity. The goal is to form a microstructure called martensite, which is the hardest and most brittle phase of steel.
The key to unlocking maximum hardness is not just the heat treatment process itself, but a combination of two critical factors: the carbon content of the steel and the cooling rate achieved during the quench.
The Core Principle: Martensitic Transformation
To understand why quenching works, you must first understand the microscopic changes happening within the steel. The entire process is designed to create a specific atomic structure that is exceptionally resistant to deformation.
What is Austenite?
At high temperatures (typically above 727°C or 1340°F), the iron atoms in steel arrange themselves into a face-centered cubic (FCC) structure called austenite. This structure has a unique ability to dissolve a significant amount of carbon atoms within its lattice.
The Role of Rapid Cooling (Quenching)
When steel is cooled slowly, the carbon atoms have time to move out of the lattice and form softer structures like pearlite.
Quenching is the act of cooling so rapidly that the carbon atoms are trapped. They don't have time to escape as the iron atoms attempt to rearrange into their room-temperature structure.
Introducing Martensite: The Hardest Microstructure
This trapping of carbon atoms forces the iron lattice into a highly strained, distorted structure known as body-centered tetragonal (BCT) martensite.
This internal strain is the source of martensite's extreme hardness and strength. The atoms are locked so tightly in this unnatural arrangement that it becomes very difficult for them to slip past one another, which is the mechanism of plastic deformation.
Key Factors Influencing Maximum Hardness
Achieving the theoretical maximum hardness for a given steel depends on controlling several key variables. Simply quenching is not enough; the details determine the outcome.
Carbon Content is King
This is the most important factor. The potential maximum hardness of a steel is almost exclusively determined by its carbon content.
A low-carbon steel (like 1018) will never become as hard as a high-carbon steel (like 1095), regardless of how perfectly it is quenched. More carbon creates more internal strain in the martensite structure, resulting in higher hardness.
The Quenching Medium Matters
The speed of cooling directly impacts whether you successfully form 100% martensite. Different liquids extract heat at different rates.
- Brine (salt water): Provides the fastest cooling rate due to the salt disrupting the formation of insulating vapor bubbles on the metal's surface.
- Water: Offers a very fast quench but can cause distortion or cracking in some steels due to its severity.
- Oil: Provides a slower, less severe quench. It is used for alloy steels that have higher "hardenability" and don't require such a rapid cooling rate.
The Importance of Hardenability
Hardenability is a measure of a steel's ability to form martensite at depth. Plain carbon steels have low hardenability and must be quenched extremely fast, making them suitable only for thin sections.
Adding alloying elements like chromium, manganese, and molybdenum increases hardenability. This allows for a slower, less severe quench (like oil) to achieve full hardness, reducing the risk of cracking and making it possible to harden thicker components.
Understanding the Trade-offs: Hardness vs. Toughness
Pursuing maximum hardness comes with a significant and often undesirable consequence. This is a critical concept that prevents catastrophic failures in engineering components.
The Brittleness of As-Quenched Martensite
Steel that has been quenched to its maximum hardness is in an "as-quenched" state. While incredibly hard and wear-resistant, it is also extremely brittle, much like glass.
This brittleness makes it unsuitable for almost all practical applications. Any sharp impact or load could cause it to shatter without warning.
The Necessity of Tempering
To make the hardened steel useful, it must undergo a secondary heat treatment called tempering. This involves reheating the part to a lower temperature (e.g., 200-650°C or 400-1200°F) and holding it for a specific time.
Tempering relieves the internal stresses within the martensite. It sacrifices a small amount of hardness but provides a significant and critical increase in toughness—the material's ability to absorb energy and resist fracture.
Making the Right Choice for Your Goal
The "best" heat treatment is entirely dependent on the final application of the component. You must define your goal before selecting a process.
- If your primary focus is maximum wear resistance and you can tolerate brittleness: Quenching a high-carbon tool steel to produce untempered martensite is the goal.
- If your primary focus is a balanced combination of strength and toughness for a structural part: Quenching followed by tempering at a specific temperature is the necessary approach.
- If your primary focus is a hard surface on a tough, ductile core: A surface treatment like case hardening (carburizing) or induction hardening is the most effective solution.
Understanding these principles allows you to precisely engineer the mechanical properties your application demands.
Summary Table:
| Factor | Role in Achieving Maximum Hardness |
|---|---|
| Quenching Process | Rapidly cools heated steel to trap carbon, forming the hard martensite microstructure. |
| Carbon Content | Determines the theoretical maximum hardness; higher carbon = greater potential hardness. |
| Cooling Rate (Quench Medium) | Faster cooling (e.g., brine) promotes more complete martensite formation. |
| Hardenability | Alloying elements allow slower quenches (e.g., oil) to achieve hardness in thicker sections. |
| Tempering | Essential follow-up process that trades a small amount of hardness for a large gain in toughness. |
Need to achieve precise hardness and toughness for your components?
KINTEK specializes in providing the advanced lab equipment and consumables necessary for controlled heat treatment processes. Whether you are developing tools, structural parts, or surface-hardened components, our solutions help you accurately manage quenching and tempering cycles.
Contact our experts today to discuss how we can support your material science and metallurgy projects with reliable, high-performance laboratory equipment.
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vertical Laboratory Tube Furnace
People Also Ask
- Why is environmental control within a vacuum furnace important for diffusion bonding? Master Titanium Alloy Laminates
- What is vacuum furnace high temperature? Unlock the Range for Your Material Processing
- Is heat Cannot travel in a vacuum True or false? Discover How Heat Crosses the Void of Space
- What is vacuum heat treatment process? Achieve Superior Control, Cleanliness, and Quality
- What is the structure of a vacuum furnace? A Guide to Its Core Components & Functions



















