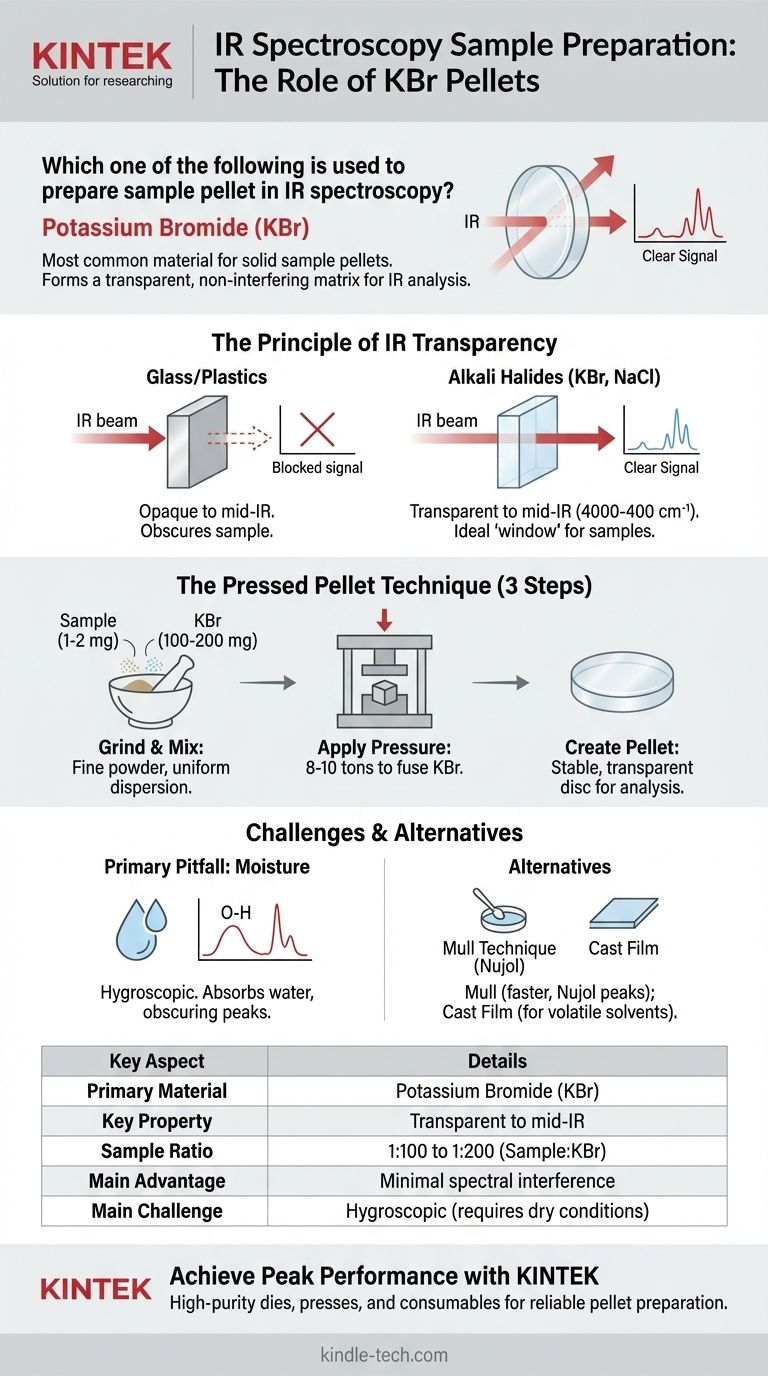
In IR spectroscopy, Potassium Bromide (KBr) is the most common material used to prepare a sample pellet. The solid sample is finely ground and mixed with a large excess of high-purity KBr powder, which is then compressed under high pressure to form a thin, transparent disc suitable for analysis.
The core challenge in analyzing solid samples via IR spectroscopy is that the material holding the sample must not interfere with the measurement. KBr is used because it is transparent to infrared radiation and can be formed into a stable, solid matrix, allowing the IR beam to pass through and interact only with the dispersed sample molecules.

The Principle of IR Transparency
Why Common Materials Fail
In spectroscopy, the sample holder or matrix must be invisible to the energy source being used.
Materials like glass, quartz, and plastics are opaque to most of the mid-infrared range. If used, they would absorb the IR radiation, and their own molecular vibrations would completely obscure the signal from the sample.
The Role of Alkali Halides
To solve this, analysts use specific inorganic salts, most often alkali halides like Potassium Bromide (KBr) or Sodium Chloride (NaCl).
These simple ionic compounds have no molecular covalent bonds that absorb energy in the most common region of the IR spectrum (4000-400 cm⁻¹). This property makes them effectively transparent to infrared light, serving as a perfect "window" to hold the sample.
A Breakdown of the Pressed Pellet Technique
Step 1: Grinding and Mixing
A very small amount of the solid sample (typically 1-2 mg) is combined with a much larger amount of dry, finely ground KBr (around 100-200 mg).
This mixture is then ground together thoroughly in an agate mortar and pestle. The goal is to reduce the sample's particle size to below the wavelength of the IR radiation to minimize light scattering and to ensure it is uniformly dispersed within the KBr.
Step 2: Applying Pressure
The powder mixture is placed into a die and compressed in a hydraulic press under immense pressure (typically 8-10 tons).
The high pressure causes the soft KBr salt to flow and fuse together, trapping the finely ground sample particles within a solid, transparent KBr matrix.
Step 3: Creating the Pellet
The result is a thin, translucent or transparent disc, known as a KBr pellet. This pellet is mechanically stable and can be placed directly into a sample holder in the spectrometer's beam path for analysis.
Common Pitfalls and Alternatives
The Primary Weakness: Moisture
The most significant drawback of KBr is that it is hygroscopic, meaning it readily absorbs moisture from the atmosphere.
If the KBr or the sample is not perfectly dry, water will be incorporated into the pellet. This will produce a large, broad absorption band for the O-H bond stretch (around 3400 cm⁻¹) in the final spectrum, which can obscure important sample peaks in that region.
Alternative Pellet Materials
While KBr is the most common choice, other IR-transparent salts like Sodium Chloride (NaCl) or Silver Chloride (AgCl) can also be used, though they are less common for the pellet technique.
Alternative Preparation: The Mull Technique
An alternative to a pressed pellet is the Mull technique. In this method, the solid sample is ground with a few drops of a mulling agent, typically a mineral oil like Nujol.
This creates a thick paste (a mull) that is then smeared between two IR-transparent salt plates (often made of NaCl or KBr). This is a faster technique but has the disadvantage that the absorption peaks of the mulling agent itself will be present in the spectrum.
Making the Right Choice for Your Sample
- If your primary focus is obtaining a high-quality, reproducible spectrum for a stable solid: The KBr pressed pellet technique is the gold standard for its clarity and lack of background interference.
- If your sample is sensitive to moisture or you need a faster preparation method: Consider the Mull technique, but be prepared to account for the Nujol peaks in your final spectrum.
- If your sample can be dissolved in a volatile solvent: The cast film technique, where a solution is evaporated on a salt plate, is an excellent and simple alternative that avoids pressure or mulling agents.
Ultimately, selecting the correct sample preparation method is fundamental to acquiring clean, interpretable, and accurate infrared spectra.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Material | Potassium Bromide (KBr) |
| Key Property | Transparent to mid-IR radiation (4000-400 cm⁻¹) |
| Sample Ratio | 1-2 mg sample to 100-200 mg KBr |
| Main Advantage | Creates a clear matrix with minimal spectral interference |
| Main Challenge | Hygroscopic; requires dry conditions to avoid water peaks |
Achieve Peak Performance in Your IR Analysis
Preparing a perfect sample pellet is critical for clear, interpretable results. KINTEK specializes in high-purity laboratory equipment and consumables, including the precise dies and hydraulic presses needed for reliable KBr pellet preparation.
Let our expertise support your laboratory's needs. Contact our team today to find the right solution for your spectroscopy workflow and ensure your samples are prepared for success.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- kbr pellet press 2t
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Lab Infrared Press Mold
People Also Ask
- What PSI should a hydraulic press be? The Answer Lies in Tonnage, Not Just Pressure
- What affects the quality of pellets? A Guide to Achieving Durable, High-Density Biomass Pellets
- What is the difference between a pneumatic press machine and a hydraulic press machine? Choose the Right Press for Your Job
- Why must ceramic powders be pressed into pellets for hardness testing? Essential Sample Preparation Insights
- Why is a laboratory high-pressure hydraulic press essential for calcium sulfate anhydrite? Achieve Uniform Green Density
- Why is a laboratory hydraulic press required for Ru/Cs+/C catalyst preparation? Optimize Density and Performance
- Why is a laboratory hydraulic press used for cold pressing SiCp/2009Al composites? Enhance Green Body Quality
- What is the difference between a manual press and a hydraulic press? Manual vs. Automatic Control Explained



















