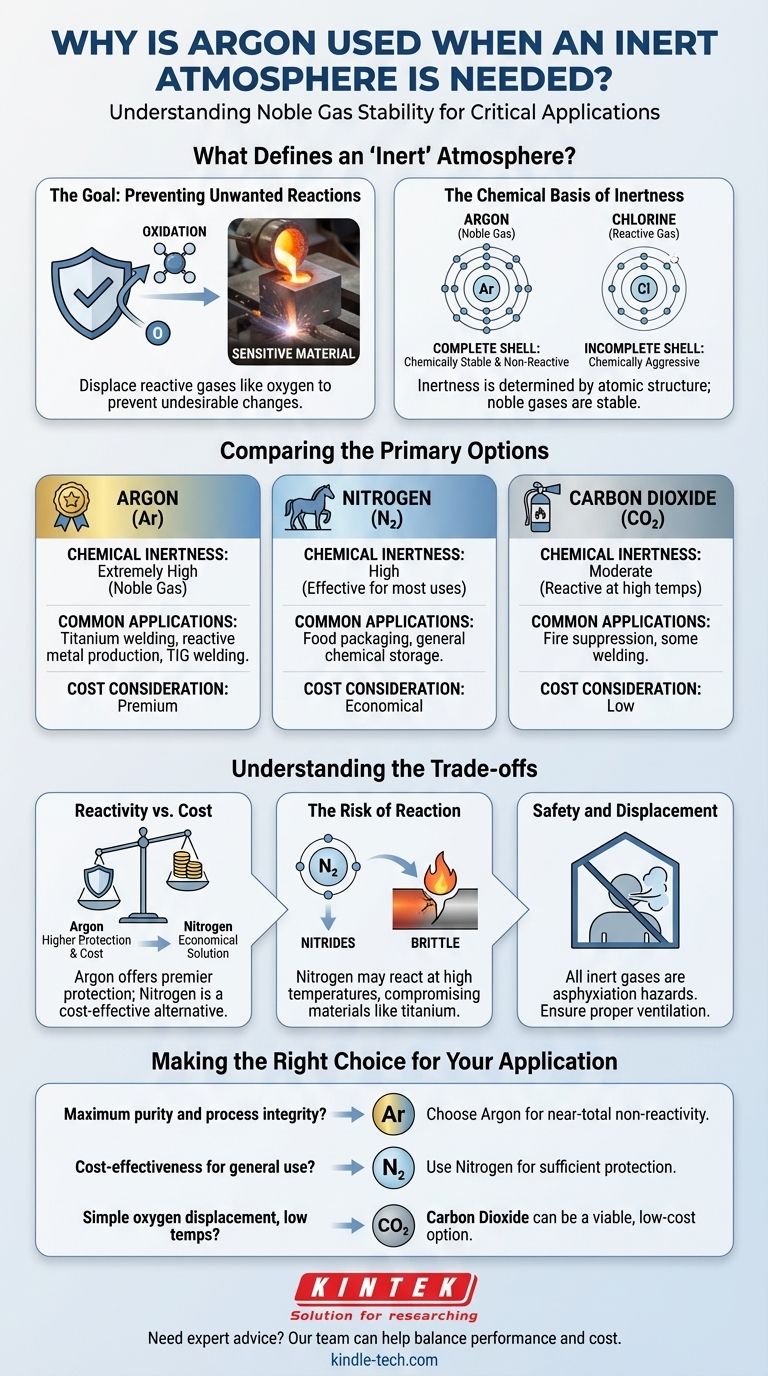
Argon is used for inert atmospheres because it is a noble gas, making it almost completely non-reactive under a vast range of temperatures and pressures. This extreme chemical stability prevents unwanted reactions like oxidation, particularly with highly sensitive or molten materials such as titanium or during high-temperature welding.
While argon offers supreme chemical inertness, the choice of an inert gas is a strategic decision. It requires balancing the required level of non-reactivity for your specific process against practical factors like cost, where gases like nitrogen often provide a more economical solution.

What Defines an "Inert" Atmosphere?
The term "inert" is not just a label; it describes a specific chemical function. Understanding this function is key to choosing the right gas for a technical application.
The Goal: Preventing Unwanted Reactions
The primary purpose of an inert atmosphere is to displace reactive gases—most commonly oxygen—to prevent undesirable chemical changes.
Think of rust on iron. That is oxidation, a slow reaction with oxygen at room temperature. At the high temperatures found in welding or metal production, these oxidative reactions happen almost instantly and can ruin the material.
An inert atmosphere creates a protective barrier, blanketing the sensitive material and preventing it from coming into contact with reactive elements in the air.
The Chemical Basis of Inertness
A gas's inertness is determined by its atomic structure. Argon is a noble gas, meaning its outermost electron shell is completely full.
This makes it chemically stable and "uninterested" in reacting with other elements. It will not readily share, gain, or lose electrons.
In contrast, a highly reactive gas like chlorine is chemically aggressive because it desperately wants to gain an electron to complete its outer shell. This is why it is an effective disinfectant but completely unsuitable for an inerting process.
Comparing the Primary Options
While argon is an excellent choice, it is not the only one. The most common gases used for inerting are argon, nitrogen, and to a lesser extent, carbon dioxide.
Argon (Ar): The Gold Standard
Argon is the premier choice when absolute non-reactivity is critical. It remains inert even under extreme conditions.
This makes it essential for high-value processes like the production of titanium and other reactive metals, as well as in specific types of high-temperature welding (e.g., TIG welding) where even minimal contamination can compromise structural integrity.
Nitrogen (N₂): The Workhorse
Nitrogen makes up about 78% of our atmosphere and is significantly cheaper to produce in pure form than argon.
Nitrogen gas exists as two atoms bonded tightly together (N₂). While this bond can be broken under very high energy conditions, it is stable enough to be considered effectively inert for a huge range of applications, from food packaging to general chemical storage.
Carbon Dioxide (CO₂): A Niche Player
Carbon dioxide is sometimes used for inerting, primarily because it is inexpensive and denser than air, allowing it to effectively displace oxygen from below.
However, CO₂ is more reactive than nitrogen or argon and can react with certain materials, especially at higher temperatures. Its use is generally limited to applications like fire suppression or some forms of welding where its reactivity is not a concern.
Understanding the Trade-offs
Selecting an inert gas is a technical decision that hinges on balancing process requirements against cost.
Reactivity vs. Cost
This is the central trade-off. Argon offers the highest level of protection and process purity, but it comes at a premium price.
Nitrogen provides a highly effective and economical solution for the majority of industrial applications where absolute inertness is not strictly required. The cost savings are often substantial.
The Risk of Reaction
The "inertness" of nitrogen is not absolute. In some high-temperature metallurgical processes, nitrogen can react with metals to form undesirable nitrides, which can make the metal brittle.
This is a key reason why argon, despite its cost, remains non-negotiable for welding or processing sensitive alloys like titanium, aluminum, and certain stainless steels.
Safety and Displacement
All inert gases, including argon and nitrogen, are asphyxiation hazards. They are colorless and odorless and work by displacing oxygen. In any enclosed or poorly ventilated space, a buildup of inert gas can reduce oxygen levels to a dangerous point, leading to loss of consciousness.
Making the Right Choice for Your Application
Your choice depends entirely on the chemical sensitivity of your process, the temperatures involved, and your budget.
- If your primary focus is maximum purity and process integrity: Choose argon for its near-total non-reactivity, especially in high-temperature metallurgy or with highly reactive elements.
- If your primary focus is cost-effectiveness for general-purpose inerting: Use nitrogen, which provides sufficient protection for a vast array of applications like food preservation and chemical blanketing.
- If your primary focus is simple oxygen displacement in a low-temperature, non-reactive setting: Carbon dioxide can be a viable, low-cost option, provided it does not react with your materials.
By understanding these core principles, you can select the appropriate gas to protect your process efficiently and effectively.
Summary Table:
| Gas | Chemical Inertness | Common Applications | Cost Consideration |
|---|---|---|---|
| Argon | Extremely High (Noble Gas) | Titanium welding, reactive metal production | Premium |
| Nitrogen | High (Effective for most uses) | Food packaging, general chemical storage | Economical |
| Carbon Dioxide | Moderate (Reactive at high temps) | Fire suppression, some welding | Low |
Need expert advice on selecting the right inert gas for your laboratory processes? At KINTEK, we specialize in providing high-purity lab equipment and consumables, including gas handling systems tailored for applications like welding, metallurgy, and chemical synthesis. Our team can help you balance performance and cost to protect your sensitive materials effectively. Contact us today to optimize your inert atmosphere setup!
Visual Guide
Related Products
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the function of a high-temperature atmosphere furnace in the heat treatment of 300M steel? Achieve Precision
- What key processing conditions does a tubular atmosphere furnace provide? Unlock Cr/SZ Catalyst Performance
- Why is an industrial furnace with hydrogen atmosphere control necessary for the pre-sintering of Fe-Cr-Al materials?
- What causes oxidation in heat treatment? Control Your Furnace Atmosphere to Prevent Scale & Decarburization
- What role does a controlled atmosphere furnace with argon gas flow play in the production of reduced graphene oxide (rGO)?
- What is the difference between a reducing atmosphere and an ordinary atmosphere? Control Oxidation for Better Results
- What is the function of a high-temperature atmosphere furnace in 20Cr-25Ni-Nb steel treatment? Expert Insights
- What is a retort furnace? A Guide to Controlled Atmosphere Heat Treatment



















