In short, a catalyst is critical in pyrolysis for two primary reasons: it significantly improves the quality of the final oil product and makes the entire conversion process more efficient. By selectively guiding chemical reactions, a catalyst allows for the production of a more valuable, stable oil that can be more easily upgraded into transportation fuels, often while reducing the energy required to run the process.
The core function of a catalyst is to transform pyrolysis from a crude thermal decomposition into a more precise chemical conversion. It acts as a control mechanism, steering the breakdown of materials like plastic or biomass toward a higher-value, more uniform final product while often lowering the required operating temperature.

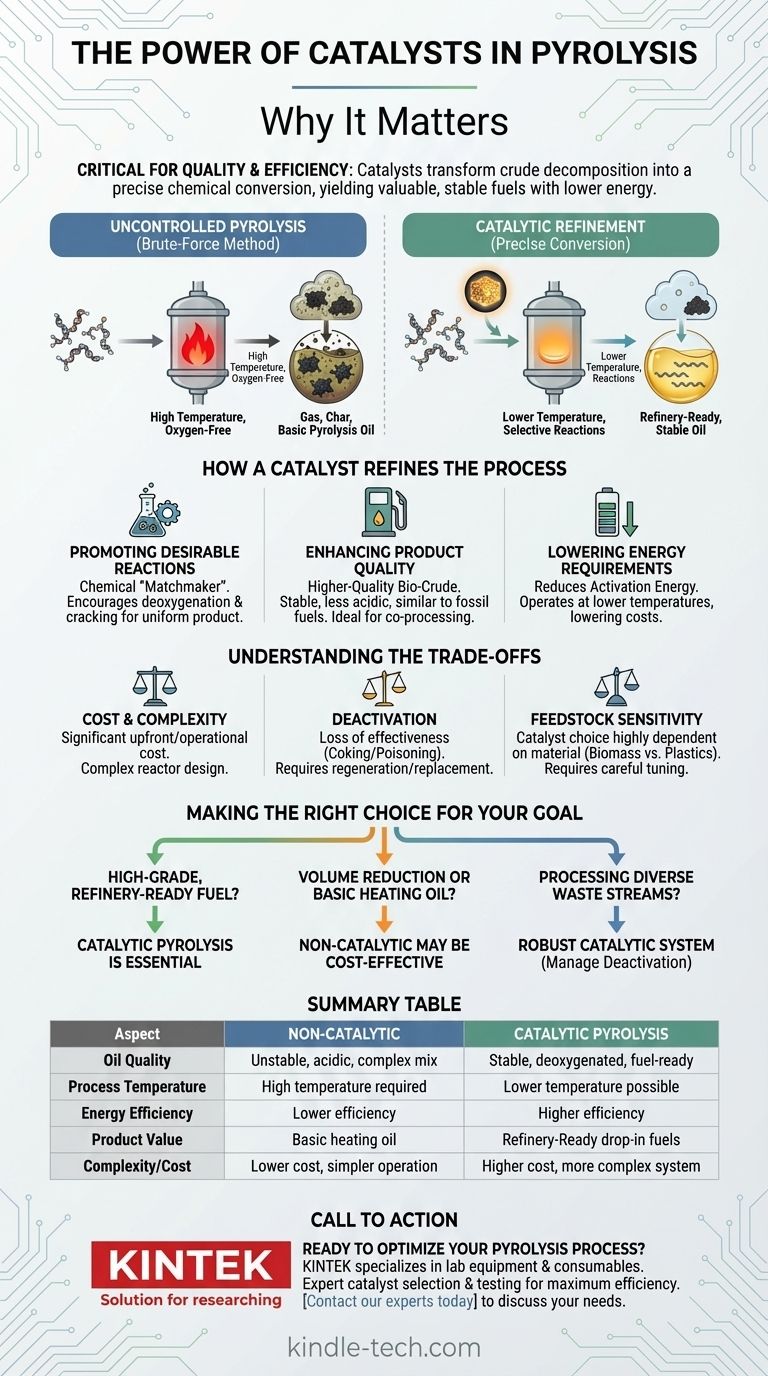
The Problem with Uncontrolled Pyrolysis
Standard, non-catalytic pyrolysis is essentially a brute-force method. It uses high heat in an oxygen-free environment to break down large, complex molecules (like plastics or biomass) into a mix of gas, a solid char, and a liquid known as pyrolysis oil.
This basic pyrolysis oil is often a complex, unstable mixture of hundreds of different chemical compounds. It can be acidic, contain high levels of oxygen, and polymerize (thicken) over time, making it difficult to store, transport, and refine.
How a Catalyst Refines the Process
Introducing a catalyst into the reactor fundamentally changes the chemical pathways of the decomposition. It provides an active surface that influences which bonds break and which new molecules form.
Promoting Desirable Chemical Reactions
A catalyst acts as a chemical "matchmaker," promoting specific reactions that are more beneficial. For example, it can encourage the removal of oxygen atoms (deoxygenation) or the cracking of long-chain hydrocarbons into the shorter chains typical of gasoline and diesel. This selectivity is the key to creating a more uniform and valuable product.
Enhancing Product Quality and Value
The direct result of this selective chemistry is a higher-quality bio-crude or pyrolysis oil. This oil is more stable, less acidic, and has a molecular composition much closer to conventional fossil fuels. This makes it a better candidate for co-processing in existing oil refineries to create drop-in fuels—fuels that are chemically indistinguishable from their petroleum-based counterparts.
Lowering Process Energy Requirements
Catalysts work by lowering the activation energy required for a chemical reaction to occur. In the context of pyrolysis, this often means the process can be run effectively at a lower temperature. Lowering the operating temperature directly translates to reduced energy consumption, which lowers operational costs and simplifies the engineering of the reactor system.
Understanding the Trade-offs
While catalysts offer significant advantages, they are not a "free lunch." Implementing a catalytic process introduces its own set of challenges.
The Cost and Complexity of Catalysts
Catalysts, especially those using specialized minerals (like zeolites) or precious metals, represent a significant upfront and operational cost. The design of the reactor must also be more complex to ensure proper contact between the feedstock vapor and the catalyst.
Catalyst Deactivation
Over time, catalysts can lose their effectiveness. This process, known as deactivation, can be caused by carbon deposits (coking) on the catalyst's surface or by poisoning from contaminants in the feedstock, such as chlorine from PVC plastic or sulfur. A deactivated catalyst must be regenerated or replaced, adding cost and operational downtime.
Feedstock Sensitivity
The choice of catalyst is not universal; it is highly dependent on the material being processed. A catalyst optimized for converting wood chips into biofuel will be different from one designed for converting mixed plastic waste into oil. This requires careful tuning and selection based on the specific feedstock and desired output.
Making the Right Choice for Your Goal
The decision to use a catalyst depends entirely on your technical and economic objectives.
- If your primary focus is producing high-grade, refinery-ready fuel: A catalytic pyrolysis process is essential to achieve the required oil quality and stability.
- If your primary focus is volume reduction or creating a basic heating oil: A simpler, non-catalytic process may be more cost-effective and operationally straightforward.
- If your primary focus is processing diverse or contaminated waste streams: A robust catalytic system can help manage impurities and improve the final product, but catalyst deactivation will be a primary concern to manage.
Ultimately, integrating a catalyst upgrades pyrolysis from a simple decomposition method into a targeted chemical manufacturing tool.
Summary Table:
| Aspect | Non-Catalytic Pyrolysis | Catalytic Pyrolysis |
|---|---|---|
| Oil Quality | Unstable, acidic, complex mix | Stable, deoxygenated, fuel-ready |
| Process Temperature | High temperature required | Lower temperature possible |
| Energy Efficiency | Lower efficiency | Higher efficiency |
| Product Value | Basic heating oil | Refinery-ready drop-in fuels |
| Complexity/Cost | Lower cost, simpler operation | Higher cost, more complex system |
Ready to optimize your pyrolysis process with the right catalyst?
KINTEK specializes in lab equipment and consumables for pyrolysis research and development. Whether you're converting biomass or plastic waste into high-value fuels, our expertise helps you select and test catalysts for maximum efficiency and product quality.
Contact our experts today to discuss your specific needs and discover how we can support your innovation in sustainable energy.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are the advantages of a wiped film evaporator? Purify Heat-Sensitive Materials Efficiently
- What is the function of an open-type reactor in SHS brass surface treatment? Achieve Precise Diffusion Saturation
- How does a laboratory magnetic stirrer contribute to pre-mixing? Master Your Photocatalytic Reaction Baselines
- What is the temperature of thermal debinding? A Guide to Controlled Binder Removal Cycles
- Which two types of heating technologies are used in heat treatments? Fuel-Fired vs. Electric Heating Explained
- What is the process of biomass conversion process? Turn Organic Waste into Energy & Fuels
- What equipment is needed for pyrolysis? The 4 Core Components for a Successful Plant
- What is the role of an incubator shaker in preparing contaminated silicone? Standardize Your Sterilization Studies



















