In electrochemical systems like batteries, voltage control is a critical safety and operational requirement. Applying an excessively high voltage must be avoided because it can cause permanent, irreversible damage by decomposing the system's electrolyte or physically harming the electrodes.
The core challenge is that voltage is both the engine of performance and a potential source of destruction. Effective voltage control is not just about preventing catastrophic failure; it is the fundamental practice that governs a system's longevity, safety, and reliability.

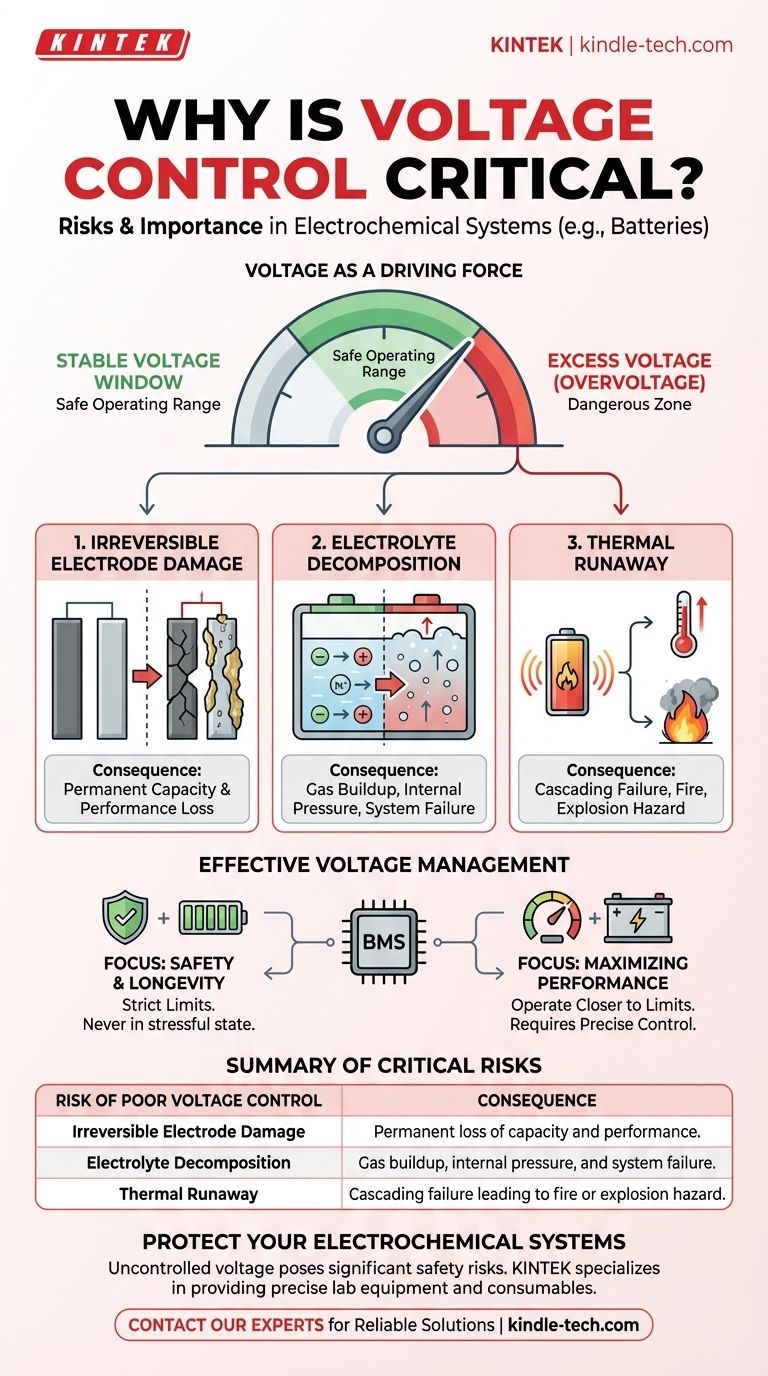
The Role of Voltage in System Health
Voltage in an electrochemical cell can be compared to pressure in a hydraulic system. It is the driving force that moves charge and enables the chemical reactions for storing and releasing energy.
Driving Desired Reactions
A specific voltage is required to make the desired chemical reactions happen efficiently. This "normal" operating voltage allows ions and electrons to move in a controlled, reversible manner, enabling processes like charging and discharging.
The Threshold of Damage
Every electrochemical system has a stable voltage window. Pushing the voltage above this window introduces excess energy that the system cannot handle constructively.
This excess energy begins to drive unintended and destructive side reactions, fundamentally altering the cell's chemistry and structure.
The Critical Risks of Poor Voltage Control
Failing to keep voltage within its designated safe operating window leads to several compounding failures. The most immediate risks involve the breakdown of the cell's core components.
Irreversible Electrode Damage
Applying excessive voltage can physically damage the electrodes. This can include fracturing the electrode material or causing unwanted plating of metallic ions, which permanently reduces the surface area available for the primary chemical reaction.
This damage directly translates to a permanent loss of capacity. The cell can no longer store or deliver the amount of energy it was designed for.
Decomposition of the Electrolyte
The electrolyte is the medium that transports ions between the electrodes. Overvoltage can break down the stable chemical compounds within the electrolyte.
This decomposition often creates gases, leading to a dangerous buildup of internal pressure. It also consumes the active electrolyte material, hindering the cell's ability to function at all.
The Danger of Thermal Runaway
These degradation processes generate heat. If the voltage is not controlled, this heat can trigger a cascading failure known as thermal runaway, where rising temperature accelerates the damaging reactions, releasing more heat.
This cycle can lead to venting of hazardous gases, fire, or even an explosion, posing a significant safety risk.
Making the Right Choice for Your System
Effective voltage management is achieved through a robust Battery Management System (BMS) or a similar controller that constantly monitors cell voltage and intervenes before it exceeds safe limits.
- If your primary focus is safety and longevity: Implement strict and conservative upper and lower voltage limits, ensuring the system never operates in a stressful state.
- If your primary focus is maximizing immediate performance: Operate closer to the established voltage limits, but this requires highly precise monitoring and control systems to prevent dangerous overshoots.
Ultimately, precise voltage control is the single most important factor in ensuring an electrochemical system operates safely and achieves its designed lifespan.
Summary Table:
| Risk of Poor Voltage Control | Consequence |
|---|---|
| Irreversible Electrode Damage | Permanent loss of capacity and performance. |
| Electrolyte Decomposition | Gas buildup, internal pressure, and system failure. |
| Thermal Runaway | Cascading failure leading to fire or explosion hazard. |
Protect your laboratory's electrochemical systems with reliable equipment. Uncontrolled voltage poses significant safety risks and can permanently damage your cells. KINTEK specializes in providing precise lab equipment and consumables to support your research and development. Ensure your experiments are safe and your results are reliable—contact our experts today to find the right solutions for your laboratory needs.
Visual Guide
Related Products
- Battery Lab Equipment Battery Capacity and Comprehensive Tester
- Manual button battery sealing machine (digital display)
- Aluminum Foil Current Collector for Lithium Battery
- Platinum Auxiliary Electrode for Laboratory Use
- Nickel Aluminum Tabs for Soft Pack Lithium Batteries
People Also Ask
- Why are customized pressure test cells necessary for ASSB testing? Master Solid-State Battery Performance
- What is the function of an in-situ spectro-electrochemical cell? Unlocking Li-CO2 Battery Reaction Insights
- What are the primary design considerations for a precision electrochemical test cell? Optimize Your Lab Characterization
- How does an electrochemical testing system evaluate mesoporous oxide electrodes? Precision Analysis for Battery Research
- What core data does a multi-channel battery test system monitor? Enhance Zinc Anode Cycling Stability Analysis



















