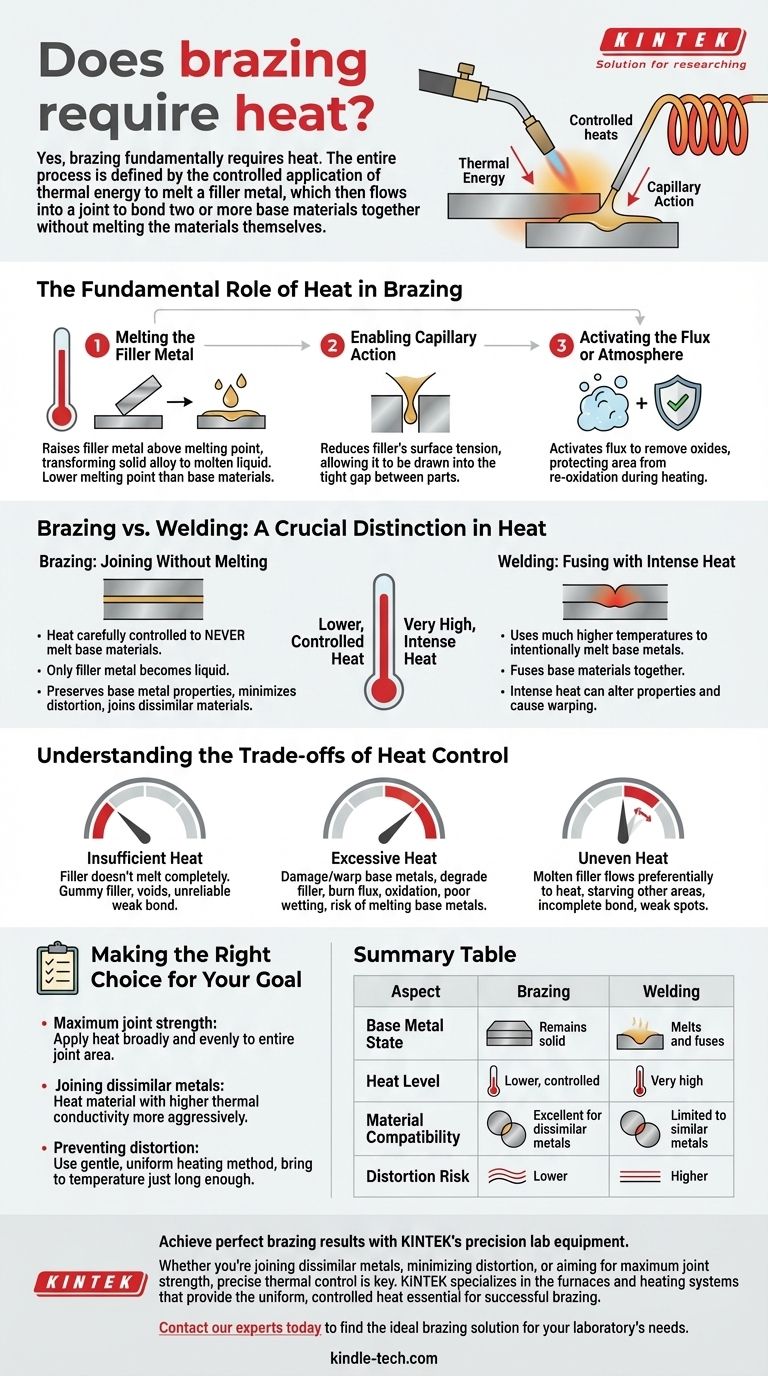
Yes, brazing fundamentally requires heat. The entire process is defined by the controlled application of thermal energy to melt a filler metal, which then flows into a joint to bond two or more base materials together without melting the materials themselves.
Brazing is not simply about applying heat; it is about precise thermal management. The goal is to heat an assembly to a specific temperature—hot enough to melt a filler alloy but cool enough to keep the base metals solid—allowing physics to create a strong, permanent bond.

The Fundamental Role of Heat in Brazing
Heat is the catalyst that enables the three critical actions of the brazing process. Without it, a brazed joint cannot be formed.
Melting the Filler Metal
The primary purpose of heat is to raise the filler metal above its specific melting temperature (its liquidus point). This transforms the solid alloy into a molten liquid.
According to metallurgical principles, this filler metal is intentionally designed to have a lower melting point than the base materials being joined.
Enabling Capillary Action
Once molten, the liquid filler metal must "wet" and flow across the surfaces of the base metals. Heat reduces the filler's surface tension, allowing it to be drawn into the tight gap between the parts through a force known as capillary action.
This capillary flow is the signature of a properly executed braze, ensuring the filler metal distributes evenly throughout the entire joint for maximum strength.
Activating the Flux or Atmosphere
In most brazing operations, a chemical flux is applied to the joint before heating. Heat activates this flux, causing it to remove oxides from the base metals and protect the area from re-oxidation during the heating cycle.
In furnace brazing, heat works with a controlled atmosphere (like hydrogen or nitrogen) to perform this same cleaning and protective function.
Brazing vs. Welding: A Crucial Distinction in Heat
Understanding how heat is used in brazing is clearest when comparing it to welding. While both join metals, their approach to thermal energy is fundamentally different.
Brazing: Joining Without Melting
In brazing, the heat is carefully controlled to never melt the base materials. Only the filler metal becomes liquid.
This lower-temperature approach preserves the original properties of the base metals, minimizes distortion, and allows for the joining of dissimilar materials (e.g., copper to steel).
Welding: Fusing with Intense Heat
Welding uses much higher temperatures to intentionally melt the edges of the base metals themselves. The molten pools of the base materials are fused together, often with the addition of a filler material.
This creates a joint that is metallurgically part of the original components, but the intense heat can alter the material properties and cause significant warping.
Understanding the Trade-offs of Heat Control
The success of a brazed joint is entirely dependent on the precise application of heat. Both insufficient and excessive heat will lead to failure.
The Risk of Insufficient Heat
If the assembly is not brought up to the correct temperature, the filler metal will not melt completely or flow properly. This results in a "gummy" filler that fails to penetrate the joint, creating voids and an unreliable, weak bond.
The Danger of Excessive Heat
Overheating is equally detrimental. It can damage or warp the base metals, degrade the metallurgical properties of the filler alloy, and burn away the protective flux prematurely, leading to oxidation and poor wetting. In extreme cases, you risk melting the base metals, which defeats the purpose of brazing.
The Problem of Uneven Heat
If one part of the joint is hotter than another, the molten filler will flow preferentially towards the heat. This can starve other areas of the joint, leading to an incomplete bond with significant weak spots.
Making the Right Choice for Your Goal
Controlling heat is the most critical skill in brazing. Your heating strategy should align directly with your desired outcome for the finished part.
- If your primary focus is maximum joint strength: Apply heat broadly and evenly to the entire joint area, encouraging the filler to be drawn uniformly through the full capillary gap.
- If your primary focus is joining dissimilar metals: Heat the material with the higher thermal conductivity more aggressively so that both sides of the joint reach the brazing temperature simultaneously.
- If your primary focus is preventing distortion: Use a gentle, uniform heating method and bring the assembly to temperature just long enough to ensure the filler flows completely.
Ultimately, heat is the essential tool that unlocks the unique metallurgical bonding process of brazing.
Summary Table:
| Aspect | Brazing | Welding |
|---|---|---|
| Base Metal State | Remains solid | Melts and fuses |
| Heat Level | Lower, controlled | Very high |
| Material Compatibility | Excellent for dissimilar metals | Limited to similar metals |
| Distortion Risk | Lower | Higher |
Achieve perfect brazing results with KINTEK's precision lab equipment.
Whether you're joining dissimilar metals, minimizing distortion, or aiming for maximum joint strength, precise thermal control is key. KINTEK specializes in the furnaces and heating systems that provide the uniform, controlled heat essential for successful brazing.
Let our expertise in lab equipment help you unlock stronger, more reliable bonds. Contact our experts today to find the ideal brazing solution for your laboratory's needs.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
People Also Ask
- Why is a blast drying oven necessary for Magnetic Fe3O4@Chitosan carbon microspheres (MCM)? Ensure Structural Integrity
- Can one furnace have multiple zones? Achieve Customized Comfort and Energy Savings
- What is the temperature of a plasma arc furnace? Achieve Extreme Heat for Advanced Materials & Waste Destruction
- What is bio-oil production and uses? A Guide to Liquid Biomass for Energy & Chemicals
- What is the function of a magnetic stirrer in sol-gel catalyst synthesis? Ensure Perfect Zeolite-Titanate Uniformity
- What are the disadvantages of sputtering process? Key Limitations in Thin-Film Deposition
- What is the significance of using an ultrasonic homogenizer to treat cells on NCD films? Optimize Protein Extraction
- What are the disadvantages of biomass to the environment? Debunking the 'Green' Myth



















