In ideal conditions, X-Ray Fluorescence (XRF) is a highly accurate and precise analytical technique. For well-prepared, homogeneous samples using specific calibrations, it's common to achieve accuracy where results are within 1-5% of the true value, with precision (repeatability) often better than 0.1%. However, this accuracy is not inherent to the instrument; it is overwhelmingly determined by the quality of your methodology.
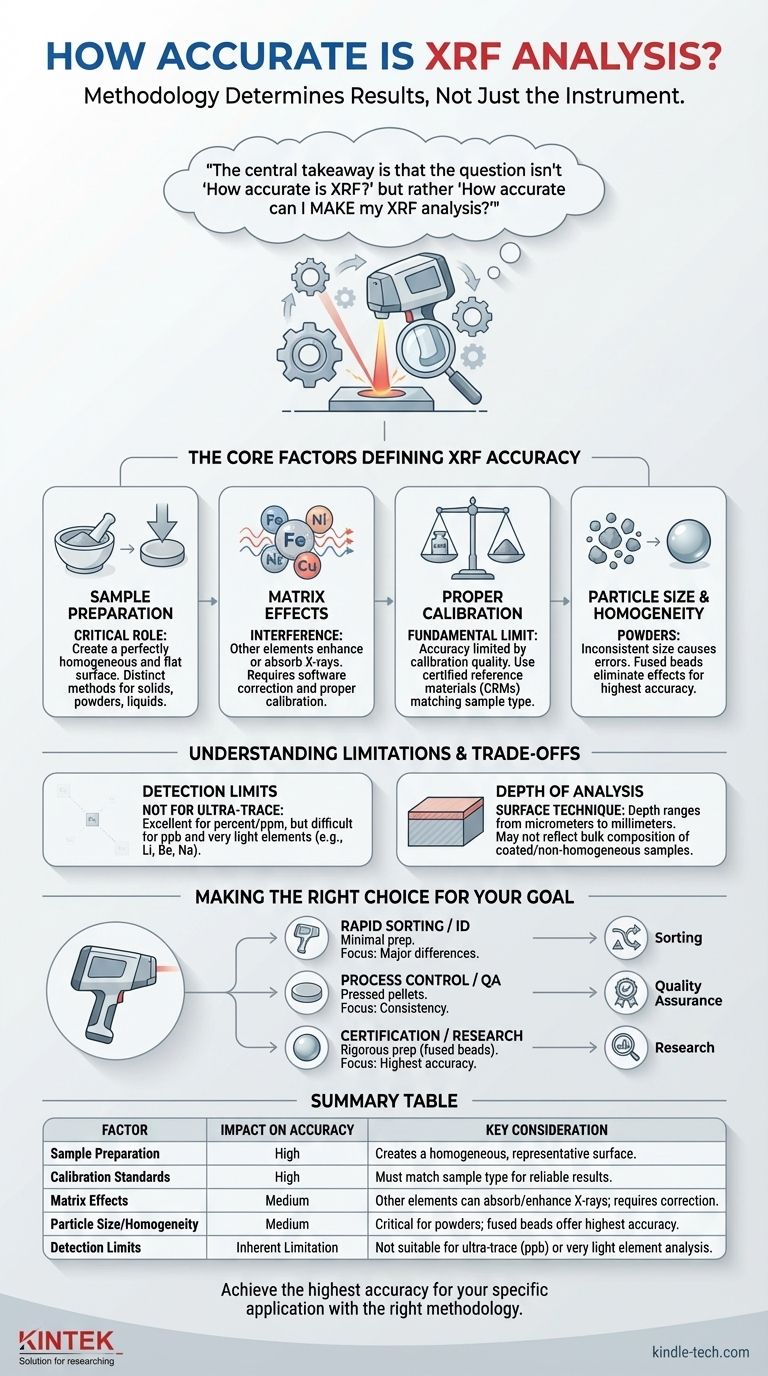
The central takeaway is that the question isn't "How accurate is XRF?" but rather "How accurate can I make my XRF analysis?" The accuracy of your results is not a fixed attribute of the machine, but a direct outcome of your sample preparation, calibration, and understanding of the sample itself.

The Core Factors Defining XRF Accuracy
The final accuracy of an XRF measurement is a function of several interdependent factors. Understanding these elements is the key to producing reliable and defensible data.
The Critical Role of Sample Preparation
This is the single most significant factor you can control. An XRF beam only analyzes a very thin layer of the sample's surface, so that surface must be perfectly representative of the entire sample.
The goal of preparation is to create a perfectly homogeneous and flat surface for the analyzer. As the provided reference indicates, different sample types (solid, powdered, liquid) require distinct preparation methods to minimize errors and ensure the measurement is reliable.
Understanding Matrix Effects
Samples are rarely composed of a single element. Matrix effects occur when the presence of other elements in the sample (the "matrix") enhances or absorbs the X-rays from the element you are trying to measure.
For example, high concentrations of iron can absorb the fluorescent X-rays from nickel, making the nickel concentration appear lower than it actually is. Modern XRF software has algorithms to correct for these effects, but they rely on proper calibration.
The Importance of Proper Calibration
An XRF instrument compares the X-ray signals from an unknown sample to signals from certified reference materials (CRMs) with known elemental concentrations. Your accuracy is fundamentally limited by the quality of your calibration.
If you are analyzing stainless steel, you must calibrate with stainless steel standards that cover the expected concentration ranges. Using a soil or plastic standard to measure a metal alloy will produce highly inaccurate results.
The Impact of Particle Size and Homogeneity
In powdered samples, inconsistent particle size can cause significant errors. Larger particles can shadow smaller ones from the X-ray beam, and different minerals may grind differently, leading to a non-representative sample surface.
This is why methods like creating fused beads (where the sample is dissolved in glass) often yield the highest accuracy for powders, as they eliminate all particle size and mineralogical effects.
Understanding the Limitations and Trade-offs
No analytical technique is perfect. Being a trusted advisor means acknowledging the areas where XRF has inherent limitations.
Detection Limits
While excellent for measuring elements in the percent or parts-per-million (ppm) range, XRF is not designed for ultra-trace analysis (parts-per-billion). Furthermore, it has difficulty detecting very light elements (like lithium, beryllium, or sodium) because their low-energy X-rays are easily absorbed and difficult to measure.
Depth of Analysis
XRF is fundamentally a surface analysis technique. The depth of penetration depends on the material and the energy of the X-rays, but it can range from a few micrometers to several millimeters.
If you are analyzing a coated, corroded, or non-homogeneous solid, the surface measurement may not reflect the bulk composition of the material. This is a common pitfall that can lead to significant misinterpretations.
The "Cost" of High Accuracy
There is a direct trade-off between speed and accuracy. A handheld XRF can provide a semi-quantitative result in seconds with no sample preparation. Achieving highly accurate, lab-quality results may require milling, pressing a pellet, or creating a fused bead, which takes significantly more time and expertise.
Making the Right Choice for Your Goal
Select your methodology based on the question you need to answer.
- If your primary focus is rapid sorting or material identification: Minimal sample preparation on a clean surface is often sufficient, as you are looking for major compositional differences, not precise percentages.
- If your primary focus is process control or routine quality assurance: Creating pressed pellets from powdered samples offers a good balance of speed and accuracy for monitoring consistency.
- If your primary focus is certification, research, or geological analysis: Rigorous sample preparation, such as creating fused beads and using type-specific calibration standards, is non-negotiable for achieving the highest possible accuracy.
Ultimately, the accuracy of your XRF analysis is a direct reflection of the quality of your methodology.
Summary Table:
| Factor | Impact on Accuracy | Key Consideration |
|---|---|---|
| Sample Preparation | High | Creates a homogeneous, representative surface for analysis. |
| Calibration Standards | High | Must match sample type (e.g., metal, soil) for reliable results. |
| Matrix Effects | Medium | Other elements can absorb or enhance X-rays; requires correction. |
| Particle Size/Homogeneity | Medium | Critical for powders; fused beads offer highest accuracy. |
| Detection Limits | Inherent Limitation | Not suitable for ultra-trace (ppb) or very light element analysis. |
Achieve the highest accuracy for your specific application. The precision of your XRF analysis is not just about the instrument—it's about the entire methodology. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the right sample preparation equipment and calibration standards to ensure your XRF results are reliable and defensible.
Contact our specialists today to discuss your analytical goals and optimize your XRF process!
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- What are the processes that need to be done after completing the sintering stage? Master the Critical Post-Sintering Steps
- What is the density of plasma matter? Exploring the Universe's Widest Range of Densities
- What are the 3 examples of heat transfer? Conduction, Convection & Radiation Explained
- What role does a Laboratory Centrifuge play in the production of succinic acid? Critical Solid-Liquid Separation Guide
- What is the unit of measurement for coating thickness? Microns (μm) and Nanometers (nm) Explained
- What are the properties of refrigerant fluids used in Ultra Freezers? Achieving Reliable -86°C Performance
- What are the applications of thin film? Powering Modern Electronics, Optics, and Energy
- What is the importance of a temperature-controlled heating device? Master the Synthesis of 1,3,4-oxadiazole Derivatives



















