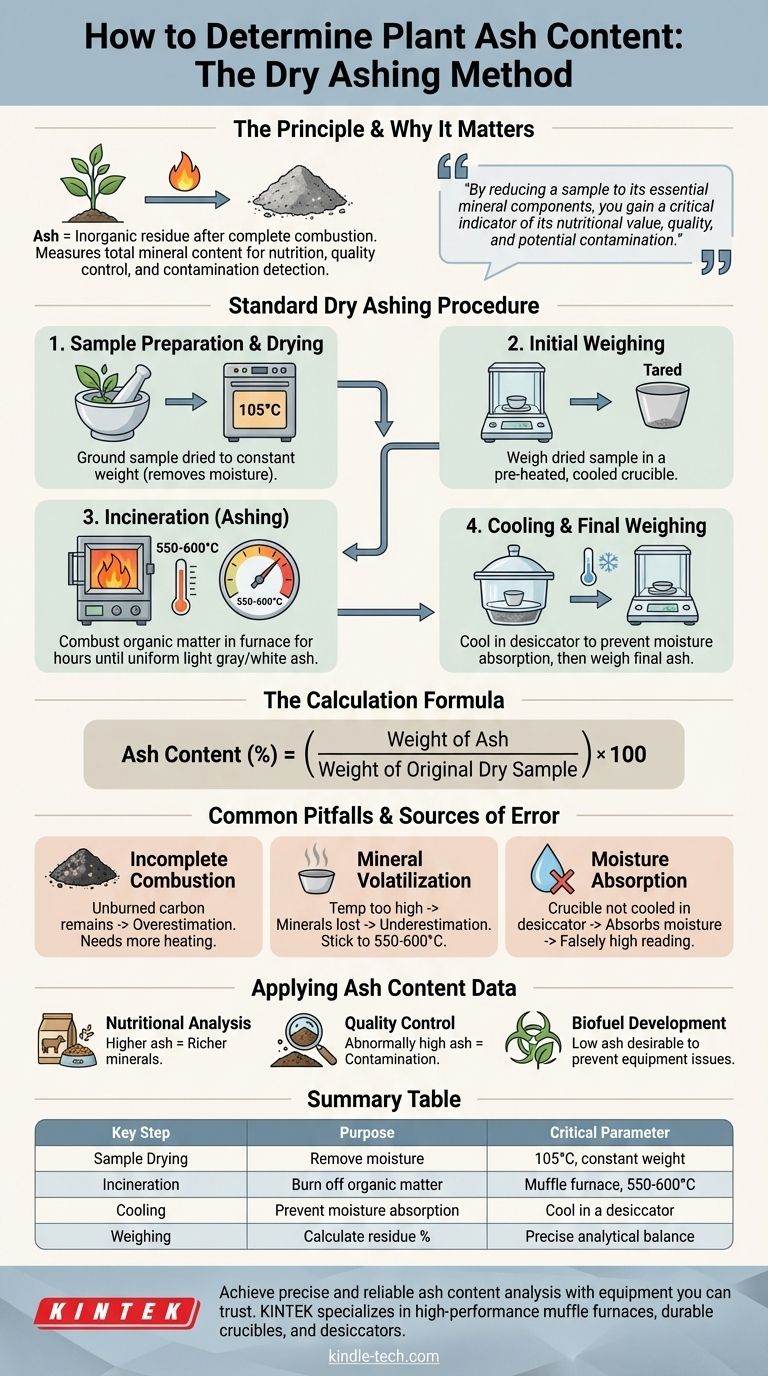
To determine the ash content of a plant sample, you must perform a procedure called dry ashing, which involves the complete combustion of the sample's organic material in a high-temperature furnace. The process measures the total amount of inorganic, noncombustible residue remaining after incineration. This residue, known as ash, represents the mineral content of the original plant material.
The core principle of ash analysis is straightforward: by reducing a sample to its essential mineral components, you gain a critical indicator of its nutritional value, quality, and potential contamination.

The Principle of Ash Content Analysis
What Ash Represents
Ash is the inorganic residue left after all organic matter—compounds containing carbon, hydrogen, and nitrogen—has been burned away. This process of high-temperature oxidation is known as incineration.
The remaining ash consists of the mineral elements present in the original plant sample. These elements, such as calcium, potassium, magnesium, and iron, are converted into their more stable oxide, sulfate, or phosphate forms during combustion.
Why It Is Measured
Measuring ash content is fundamental in many fields. In food science and animal nutrition, it provides a direct measure of the total mineral content. In quality control, an unusually high ash value can indicate contamination with soil or sand.
The Standard Laboratory Procedure
A precise and consistent methodology is crucial for obtaining accurate results. The following steps outline the standard dry ashing technique.
Step 1: Sample Preparation and Drying
Before ashing, the sample must be homogenous and free of moisture. Water content would add weight and lead to inaccurate results.
The plant sample is typically ground into a fine powder and dried in an oven at 105°C until it reaches a constant weight. This ensures the starting point for the calculation is based purely on the dry matter.
Step 2: Initial Weighing
An empty crucible, made of porcelain or another heat-resistant material, is heated to the ashing temperature, cooled in a desiccator, and weighed accurately. This pre-heating and cooling cycle ensures that any moisture or volatile residue on the crucible is removed.
A known mass of the dried plant sample (typically 1-5 grams) is then placed into this tared crucible and weighed again with high precision.
Step 3: Incineration (Ashing)
The crucible containing the sample is placed into a muffle furnace. The temperature is gradually increased to the target, usually between 550°C and 600°C.
This high temperature ensures the complete oxidation and volatilization of all organic components. The sample is left in the furnace for several hours, or until the residue becomes a uniform, light gray or white color, indicating that all carbon has been burned off.
Step 4: Cooling and Final Weighing
The crucible is carefully removed from the furnace and placed into a desiccator. The desiccator contains a drying agent that prevents the highly hygroscopic (water-absorbing) ash from absorbing moisture from the air as it cools.
Once it has returned to room temperature, the crucible containing the ash is weighed one final time.
The Calculation
The ash content is expressed as a percentage of the original dry sample weight. The calculation is simple:
Ash Content (%) = (Weight of Ash / Weight of Original Dry Sample) * 100
Common Pitfalls and Sources of Error
Achieving accurate ash content data requires careful attention to detail. Several factors can compromise the integrity of the results.
Incomplete Combustion
If the final ash is dark or contains black specks, it signifies that unburned carbon remains. This leads to an overestimation of the ash content. The solution is to return the sample to the muffle furnace for additional heating until the ash is a consistent light color.
Mineral Volatilization
Using a furnace temperature that is too high can cause certain minerals (such as chlorides and nitrates) to vaporize and be lost. This leads to an underestimation of the true ash content. Sticking to the validated temperature range of 550-600°C is critical for most plant materials.
Moisture Absorption
Ash is extremely hygroscopic. If the crucible is not cooled completely within a desiccator, it will absorb atmospheric moisture, adding weight and causing a falsely high ash reading. This is one of the most common sources of error.
Applying Ash Content Data to Your Goal
The final percentage is more than just a number; it provides actionable insight depending on your objective.
- If your primary focus is nutritional analysis: A higher ash content generally indicates a richer source of essential minerals, a key quality metric for food and animal feed.
- If your primary focus is quality control: An abnormally high ash value can signal adulteration with inorganic materials like sand, soil, or dust.
- If your primary focus is biofuel development: Low ash content is highly desirable, as minerals can cause slagging, fouling, and corrosion in combustion equipment.
Mastering this fundamental technique provides a reliable window into the inorganic composition of any plant material.
Summary Table:
| Key Step | Purpose | Critical Parameter |
|---|---|---|
| Sample Drying | Remove moisture for accurate baseline weight | 105°C until constant weight |
| Incineration | Burn off all organic matter | Muffle furnace at 550-600°C |
| Cooling | Prevent ash from absorbing atmospheric moisture | Cool in a desiccator |
| Weighing | Calculate the percentage of inorganic residue | Use a precise analytical balance |
Achieve precise and reliable ash content analysis with equipment you can trust.
The accuracy of your mineral analysis depends entirely on the precision of your furnace and lab tools. KINTEK specializes in high-performance muffle furnaces, durable crucibles, and desiccators designed for the rigorous demands of dry ashing.
We help laboratories like yours:
- Ensure complete combustion with uniform, stable furnace temperatures.
- Prevent mineral loss or moisture absorption with reliable equipment.
- Generate consistent, high-quality data for nutrition, quality control, and research.
Ready to enhance your lab's capabilities? Contact our experts today to find the perfect solution for your ash analysis workflow.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is a high-temperature solution annealing furnace necessary for HT-UPS steel? Unlock Peak Material Performance
- Why Use a Muffle Furnace for Post-Annealing Mg-Doped NASICON? Boost Electrolyte Density to 98% and Ionic Conductivity
- Why is laboratory heating equipment necessary for P@S glue? Essential 100°C Thermal Control for Electrolyte Prep
- What is the purpose of using a high-temperature muffle furnace in the preparation of Cr–Mn doped TiO2 nanowires?
- What is the significance of sintering? Unlock Strong, Complex Parts Without Melting
- What role does a high-temperature box furnace play in the solution treatment of Nickel-based 625 alloy?
- What is the function of a laboratory high-temperature furnace during LCFA calcination? Achieve Pure Perovskite Oxides
- What is the function of a high-temperature muffle furnace in eggshell calcination? Achieve 900°C Precise Conversion



















