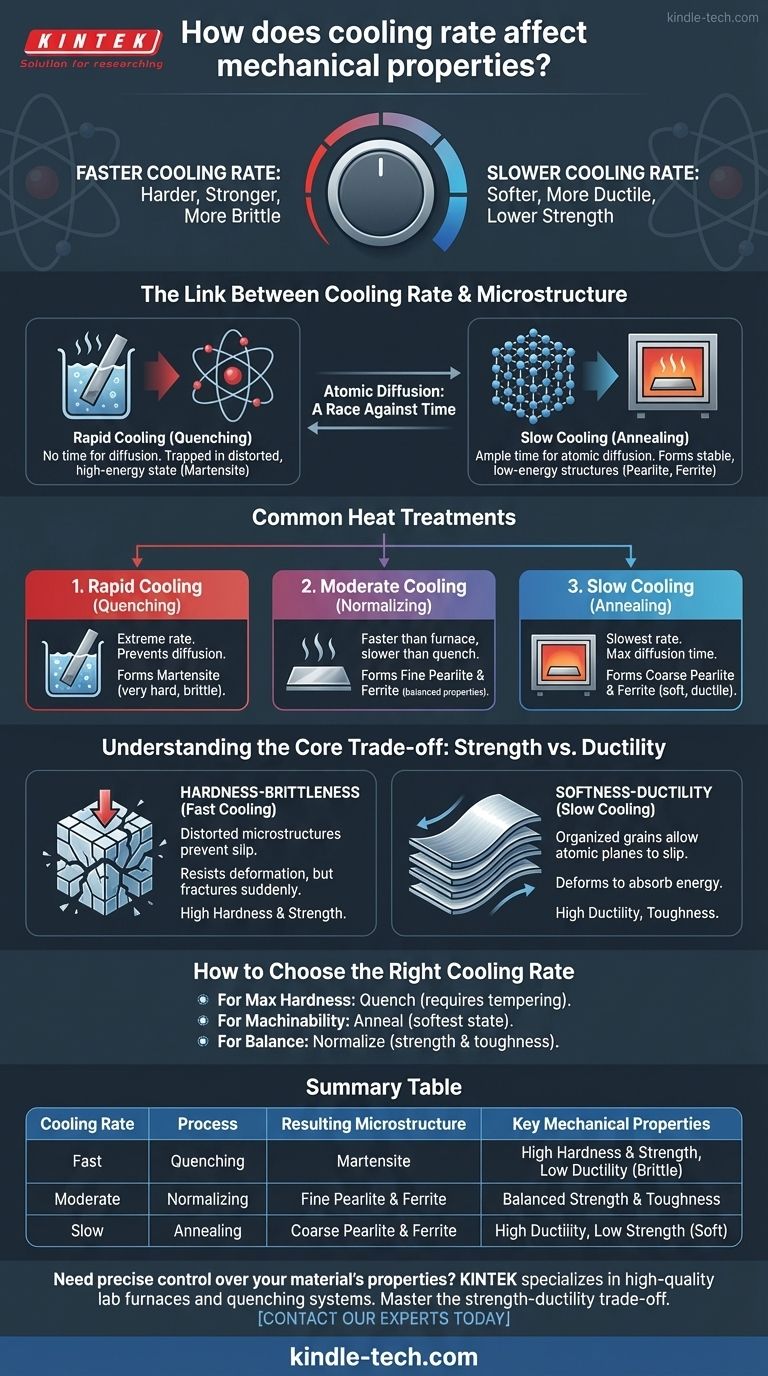
In materials science, cooling rate is the primary control knob for a material's final mechanical properties. In heat-treatable alloys like steel, a faster cooling rate generally produces a material that is harder and stronger, but also more brittle. Conversely, a slower cooling rate results in a softer, more ductile material with lower strength.
The speed at which an alloy is cooled from a high temperature directly dictates its internal crystal structure, known as its microstructure. This creates a fundamental and predictable trade-off: you can optimize for strength and hardness or for ductility and toughness, but you cannot maximize both through cooling rate alone.

The Link Between Cooling Rate and Microstructure
To understand how cooling rate impacts properties, we must first look at what happens inside the material at an atomic level. The arrangement of atoms into different crystal structures, or phases, is what gives a material its unique characteristics.
Why Microstructure Dictates Properties
Mechanical properties like strength and ductility are not inherent to a chemical composition; they emerge from the material's microstructure. A structure that resists the internal slipping of atomic planes will be hard and strong. A structure that allows for this slip will be soft and ductile.
The Role of Atomic Diffusion
Heat treatment processes begin by heating an alloy until it forms a single, uniform solid phase (like austenite in steel). The cooling process that follows is a race against time for the atoms to rearrange themselves into new, stable phases.
Slower cooling provides ample time for atomic diffusion, allowing atoms to move and organize into soft, stable, low-energy structures. Rapid cooling denies atoms this time, trapping them in a distorted, high-energy, and highly-strained state.
Common Heat Treatments and Their Effects
The rate of cooling is the defining variable in the three most common heat treatments for steel: quenching, normalizing, and annealing.
Rapid Cooling (Quenching)
Quenching involves cooling the material as rapidly as possible by submerging it in a medium like water, oil, or brine.
This extreme cooling rate prevents normal atomic diffusion. In steel, it forces the formation of a microstructure called martensite, a body-centered tetragonal structure. This structure is highly strained, extremely hard, and very strong, but it is also exceptionally brittle.
Moderate Cooling (Normalizing)
Normalizing involves cooling the material in still air. This is faster than furnace cooling but much slower than quenching.
This rate allows for some diffusion, resulting in a fine-grained microstructure of pearlite and ferrite. This refined structure provides a good balance of properties: stronger and harder than an annealed state, yet more ductile and tougher than a quenched state.
Slow Cooling (Annealing)
Annealing is the slowest process, where the material is often left to cool down inside a turned-off furnace over many hours.
This maximal time for diffusion allows the atoms to form a coarse-grained, low-stress microstructure. The resulting material is in its softest, weakest, and most ductile state, making it easy to machine or form.
Understanding the Core Trade-off: Strength vs. Ductility
The relationship between cooling rate and mechanical properties is governed by a fundamental trade-off. Improving one property often comes at the expense of another.
The Hardness-Brittleness Correlation
The distorted, high-stress microstructures like martensite, formed by fast cooling, are very effective at preventing the internal atomic slip that constitutes plastic deformation. This makes them incredibly hard and strong.
However, this same resistance to deformation means that when the material is overloaded, it has no mechanism to deform and absorb energy. Instead, it fractures suddenly, which is the definition of brittleness.
The Softness-Ductility Relationship
The stable, low-stress microstructures formed by slow cooling have neatly organized crystal grains that allow atomic planes to slip past one another relatively easily. This makes the material soft and reduces its overall strength.
This ability to deform internally is what defines ductility. It allows the material to bend, stretch, and absorb significant energy before fracturing, making it tougher and more forgiving in many applications.
How to Choose the Right Cooling Rate
Selecting the appropriate cooling rate is not about finding the "best" one, but about achieving the specific properties required for an application.
- If your primary focus is maximum hardness: Quench to form martensite, but understand this almost always requires a secondary tempering process to restore some toughness.
- If your primary focus is machinability and stress relief: Anneal to achieve the softest and most ductile state possible.
- If your primary focus is a balanced and refined material: Normalize to create a uniform, fine-grained structure with a good combination of strength and toughness.
Ultimately, mastering the cooling rate is fundamental to engineering materials to meet precise performance demands.
Summary Table:
| Cooling Rate | Process | Resulting Microstructure (in Steel) | Key Mechanical Properties |
|---|---|---|---|
| Fast | Quenching | Martensite | High Hardness & Strength, Low Ductility (Brittle) |
| Moderate | Normalizing | Fine Pearlite & Ferrite | Balanced Strength & Toughness |
| Slow | Annealing | Coarse Pearlite & Ferrite | High Ductility, Low Strength (Soft) |
Need precise control over your material's properties? The right lab equipment is crucial for achieving accurate cooling rates and reliable results. KINTEK specializes in high-quality lab furnaces and quenching systems designed for consistent heat treatment. Whether you're developing new alloys or ensuring quality control, our solutions help you master the strength-ductility trade-off. Contact our experts today to find the perfect heat treatment equipment for your laboratory's needs.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
People Also Ask
- How hot does a muffle furnace get? Unlock the Right Temperature for Your Lab
- What is a muffle furnace used to measure? Unlock Precise Sample Analysis with High-Temp Heating
- What is the point of a muffle? Achieve Purity and Precision in High-Temperature Processes
- What is the operating temperature of a muffle furnace? From 200°C to 1800°C for Your Application
- What is a muffle furnace and how does it work? Achieve Clean, High-Temperature Heating for Your Lab



















