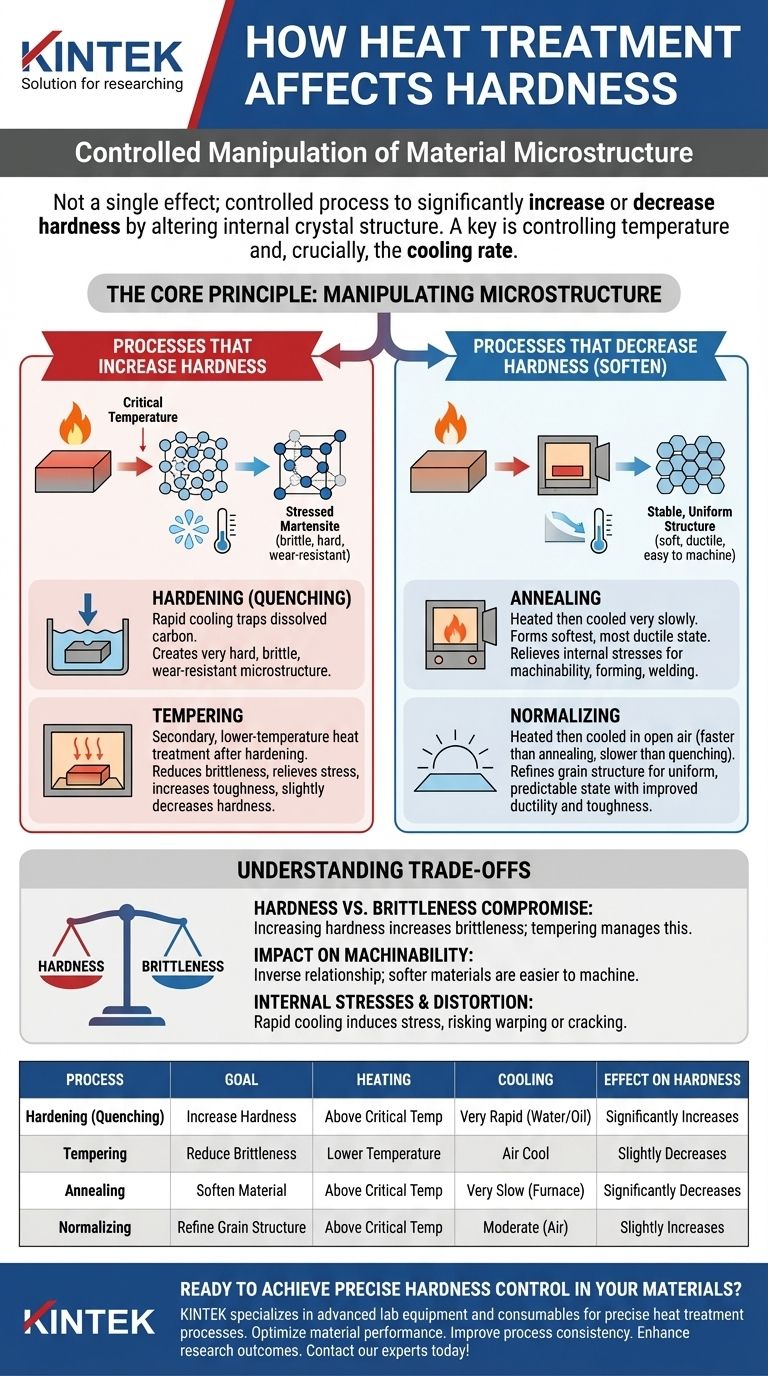
To be clear, heat treatment does not have a single effect on hardness. It is a controlled process that can be used to either significantly increase or decrease the hardness of a material, depending entirely on the specific heating and cooling cycle applied. The goal is to deliberately alter the material's internal crystal structure to achieve a desired set of mechanical properties.
The core principle is that heat treatment is not a side effect; it is an intentional manipulation. By controlling the temperature and, most importantly, the cooling rate, you are fundamentally reorganizing the material's atomic structure to make it harder and more wear-resistant or softer and more machinable.

The Core Principle: Manipulating Microstructure
The hardness of a metal is determined by its microstructure, which is the arrangement of its atoms into crystal grains. Heat treatment works by using thermal energy to unlock and rearrange this internal structure.
How Heating Changes the Structure
When a metal like steel is heated above a specific critical temperature, its atoms rearrange into a new crystal structure (austenite) that can dissolve elements like carbon. This creates a uniform, solid solution, resetting the material's internal state.
The Critical Role of Cooling Rate
The true transformation happens during cooling. The speed of cooling dictates what kind of microstructure forms as the metal returns to a lower temperature, which in turn determines its final hardness and other mechanical properties.
Processes that Increase Hardness
To make a material harder, the goal is to trap its atomic structure in a highly stressed, disordered state.
Hardening (Quenching)
Hardening involves heating the material to its critical temperature and then cooling it very rapidly. This process is often called quenching and is typically done by submerging the hot part in water, oil, or another medium.
This rapid cooling traps the dissolved carbon atoms, creating a very hard, brittle, and wear-resistant microstructure known as martensite.
Tempering
A quenched part is often too brittle for practical use. Tempering is a secondary, lower-temperature heat treatment applied after hardening.
It slightly reduces the hardness and wear resistance but significantly decreases brittleness and relieves the internal stresses caused by quenching, resulting in a much tougher final component.
Processes that Decrease Hardness (Soften)
To make a material softer, the goal is to allow its atoms to form a stable, uniform, and stress-free structure.
Annealing
Annealing is the process of heating a material and then cooling it as slowly as possible. This slow cool allows the microstructure to form into its softest, most ductile state.
This process relieves internal stresses and is primarily used to make a material easier to machine, form, or weld.
Normalizing
Normalizing involves heating the material and then letting it cool in open air. The cooling is faster than annealing but much slower than quenching.
This refines the grain structure, producing a material that is slightly harder than an annealed one but with improved ductility and toughness. It creates a more uniform and predictable mechanical state.
Understanding the Trade-offs
Choosing a heat treatment process is always an exercise in balancing competing properties. It is impossible to maximize all desirable traits simultaneously.
The Hardness vs. Brittleness Compromise
The most fundamental trade-off is between hardness and brittleness. As you increase a material's hardness, you almost always increase its brittleness, making it more susceptible to fracturing under sharp impacts. Tempering is the primary method used to manage this compromise.
Impact on Machinability
A material's hardness has a direct and inverse relationship with its machinability. Soft, annealed materials are easy to cut, drill, and shape. Fully hardened materials can be extremely difficult or even impossible to machine with traditional tools.
Internal Stresses and Distortion
Rapid cooling cycles like quenching induce significant internal stresses within a material. If not properly managed, these stresses can cause the part to warp, distort, or even crack during or after the heat treatment process.
Making the Right Choice for Your Goal
Select the heat treatment process based on the final performance requirements of the component.
- If your primary focus is maximum wear resistance and strength: Use hardening (quenching) to create a hard martensitic structure, followed by tempering to reduce brittleness to an acceptable level.
- If your primary focus is improving machinability or formability: Use annealing to put the material in its softest, most ductile, and stress-free state before manufacturing operations.
- If your primary focus is refining grain structure after forging or forming: Use normalizing to create a uniform and consistent microstructure, improving the overall toughness of the part.
Ultimately, heat treatment gives you direct control over a material's mechanical destiny.
Summary Table:
| Process | Goal | Heating | Cooling | Effect on Hardness |
|---|---|---|---|---|
| Hardening (Quenching) | Increase Hardness | Above Critical Temp | Very Rapid (Water/Oil) | Significantly Increases |
| Tempering | Reduce Brittleness | Lower Temperature | Air Cool | Slightly Decreases |
| Annealing | Soften Material | Above Critical Temp | Very Slow (Furnace) | Significantly Decreases |
| Normalizing | Refine Grain Structure | Above Critical Temp | Moderate (Air) | Slightly Increases |
Ready to achieve precise hardness control in your materials?
At KINTEK, we specialize in providing advanced lab equipment and consumables for precise heat treatment processes. Whether you're working on hardening, tempering, or annealing, our solutions help you:
• Optimize material performance with precise temperature control • Improve process consistency with reliable laboratory equipment • Enhance research outcomes with specialized heat treatment consumables
Let's discuss your specific laboratory needs – Contact our experts today to find the perfect heat treatment solution for your application!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How to use a muffle furnace in a laboratory? A Step-by-Step Guide to Safe, Precise Thermal Processing
- What are the precautions of muffle furnace? Essential Safety Protocols for Your Lab
- What happens after calcination? A Guide to Material Transformation and Next Steps
- What is the theory of muffle furnace? Achieve Pure, Controlled High-Temperature Processing
- How does tempering reduce hardness? Achieve the Perfect Balance of Toughness and Durability



















