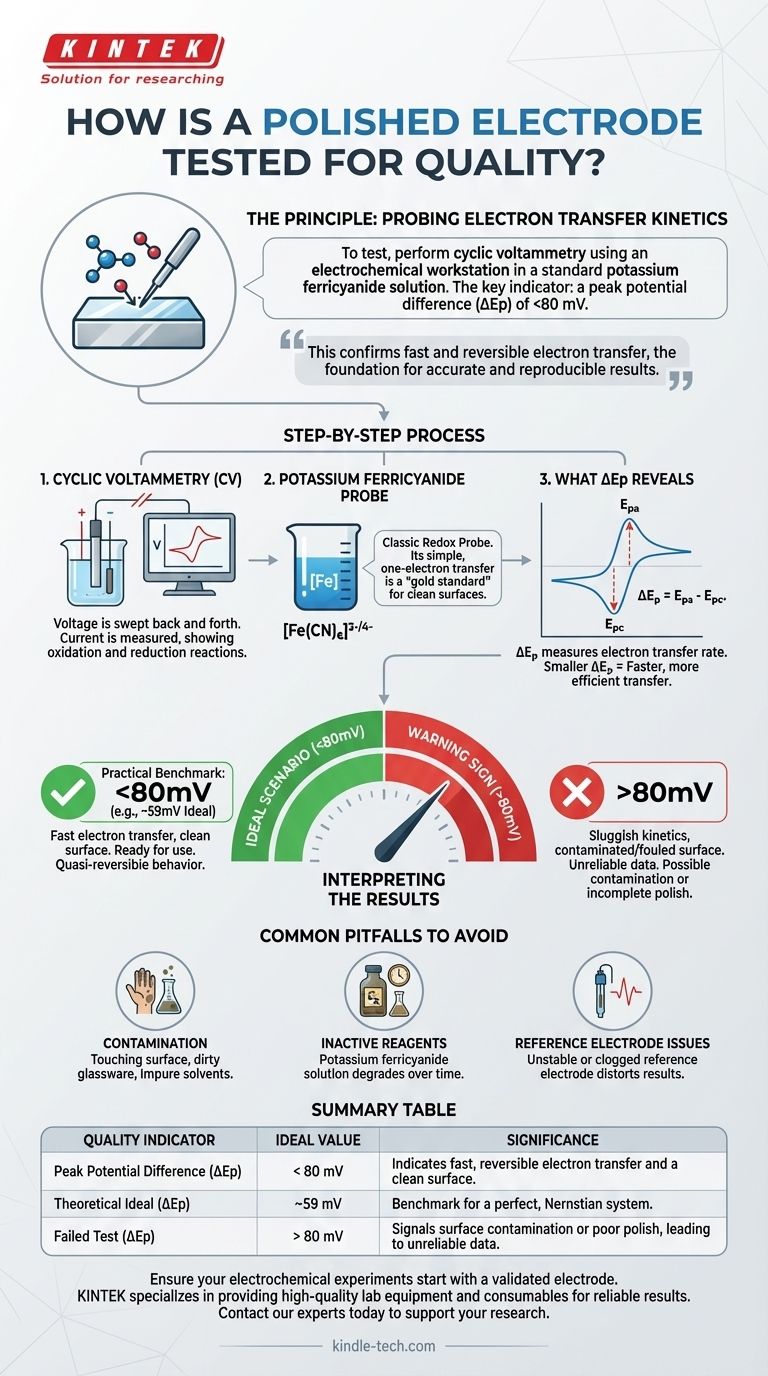
To test the quality of a polished electrode, you perform cyclic voltammetry using an electrochemical workstation. This test is run in a standard potassium ferricyanide solution, which acts as a known benchmark. The key indicator of a successful polish is a peak potential difference of less than 80 millivolts (mV).
The core principle is not just about cleanliness; it's about verifying the electrode's electrochemical performance. This test confirms that the polished surface allows for fast and reversible electron transfer, which is the foundation for accurate and reproducible experimental results.

The Principle: Probing Electron Transfer Kinetics
A quality check on a polished electrode is fundamentally a test of its surface's ability to facilitate a chemical reaction. We use a well-understood reaction to probe the unknown state of our electrode surface.
What is Cyclic Voltammetry (CV)?
Cyclic voltammetry is a technique where the voltage applied to the electrode is swept back and forth between two set points. As the voltage changes, we measure the current that flows. This current corresponds to chemical oxidation and reduction reactions occurring at the electrode surface.
The resulting plot of current versus voltage, called a voltammogram, provides a direct diagnostic of the electrode's behavior.
Why Potassium Ferricyanide?
Potassium ferricyanide ([Fe(CN)₆]³⁻/⁴⁻) is used because it is a classic redox probe. Its reaction is a simple, one-electron transfer that is known to be highly reversible and fast on a clean electrode surface.
By using this predictable "gold standard" system, any slowness or deviation we observe can be attributed directly to the quality of our electrode surface.
What Peak Potential Difference (ΔEp) Reveals
During the CV scan, we see a current peak for the oxidation reaction (anodic peak, Epa) and another for the reduction reaction (cathodic peak, Epc).
The peak potential difference (ΔEp) is the voltage separation between these two peaks (ΔEp = Epa - Epc). This value is a direct measure of the rate of electron transfer at the electrode's surface. A smaller ΔEp means faster, more efficient electron transfer.
Interpreting the Results: What the Voltage Tells You
The entire test hinges on comparing your measured peak separation to the theoretical ideal. This comparison immediately tells you if your electrode is ready for an experiment.
The Ideal Scenario: Nernstian Behavior
For a theoretically perfect, infinitely fast one-electron transfer, the peak separation (ΔEp) would be approximately 59 mV at room temperature. This is known as ideal "Nernstian" or reversible behavior.
The Practical Benchmark: Less Than 80mV
In practice, achieving the exact theoretical value is rare. A ΔEp value within 80 mV is widely accepted as the standard for a well-polished electrode exhibiting "quasi-reversible" behavior.
This indicates that the electron transfer kinetics are fast and not being hindered by surface contaminants, oxides, or defects from a poor polish. The electrode is considered ready for use.
The Warning Sign: A High ΔEp (>80mV)
If the measured ΔEp is significantly greater than 80 mV, it signals sluggish electron transfer kinetics.
This is a clear indicator that the electrode surface is contaminated, incompletely cleaned, or fouled. Using an electrode in this state would lead to inaccurate and unreliable data, as your measurements would be limited by the poor performance of the electrode itself.
Common Pitfalls to Avoid
A failed quality check doesn't always mean you need to polish again. Sometimes the issue lies elsewhere in the process.
Contamination After Polishing
A perfectly polished surface is highly active and easily contaminated. Touching the surface, using dirty glassware, or rinsing with impure solvents can ruin the preparation and lead to a high ΔEp.
Inactive Reagents
The potassium ferricyanide solution can degrade over time. If you consistently get poor results with well-polished electrodes, the test solution itself may be the culprit.
Reference Electrode Issues
An unstable or clogged reference electrode can also distort the cyclic voltammogram and give a false impression of poor working electrode performance. Always ensure all components of your electrochemical cell are in good condition.
Making the Right Choice for Your Goal
This simple CV test is not just a procedural step; it is the fundamental validation of your most critical tool.
- If your primary focus is quantitative analysis: Achieving a low and stable ΔEp is non-negotiable, as it ensures your measurements are accurate and not skewed by poor electrode kinetics.
- If you are troubleshooting a failing experiment: This CV check should be your first diagnostic step to confirm or eliminate the working electrode as the source of the problem.
- If you are developing new sensors or materials: Using this standard test provides an essential baseline to compare the performance of your modified electrode against a clean, ideal surface.
Mastering this quality check is the foundation for obtaining reliable and reproducible electrochemical data.
Summary Table:
| Quality Indicator | Ideal Value | Significance |
|---|---|---|
| Peak Potential Difference (ΔEp) | < 80 mV | Indicates fast, reversible electron transfer and a clean surface. |
| Theoretical Ideal (ΔEp) | ~59 mV | Benchmark for a perfect, Nernstian system. |
| Failed Test (ΔEp) | > 80 mV | Signals surface contamination or poor polish, leading to unreliable data. |
Ensure your electrochemical experiments start with a validated electrode.
KINTEK specializes in providing the high-quality lab equipment and consumables you need for reliable results, from electrochemical workstations to polishing supplies. A properly tested electrode is the foundation of accurate data.
Contact our experts today to discuss your laboratory needs and how we can support your research with the right tools for success.
Visual Guide
Related Products
- Metal Disc Electrode Electrochemical Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
People Also Ask
- What is the expected lifespan of a metal disk electrode? Extend Its Life with Proper Care
- What materials can be used for metal disk electrodes? Selecting the Right Metal for Your Electrochemical Experiment
- What is the common role of a platinum disk electrode? A Guide to Its Primary Use as a Working Electrode
- What is the purpose of selecting polycrystalline disc electrodes? Achieve Precision in Noble Metal Corrosion Research
- What are the key performance characteristics of a metal disk electrode? Ensuring Accurate Electrochemical Measurements



















