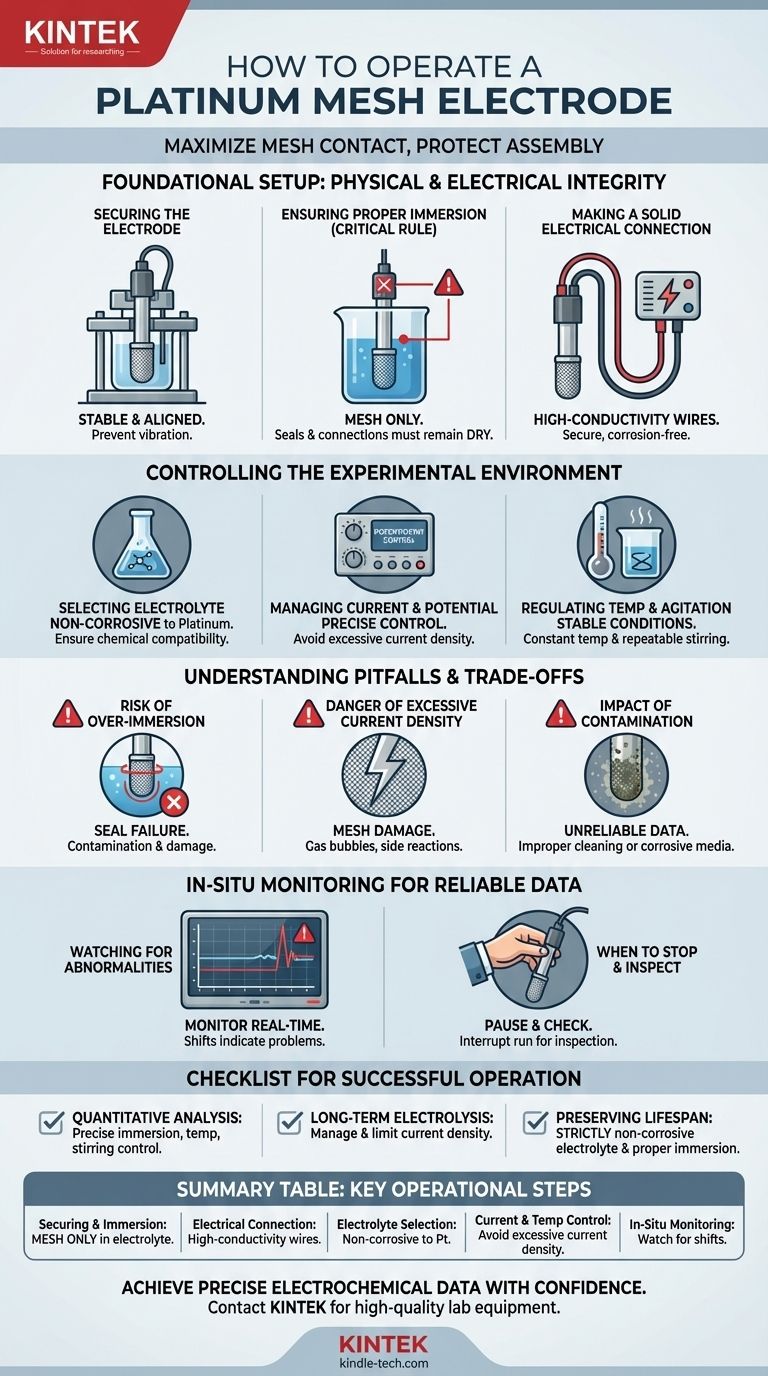
To operate a platinum mesh electrode correctly, you must first secure it within your electrochemical cell, ensuring only the platinum mesh itself is immersed in the electrolyte. Connect it to your potentiostat or power source with high-conductivity wires, then select an electrolyte that is non-corrosive to platinum. During the experiment, carefully control the current density and temperature to prevent damage and ensure accurate, repeatable results.
The central principle of using a platinum mesh electrode is maximizing the contact between the active mesh surface and the electrolyte while rigorously protecting all other parts of the electrode assembly from immersion. This discipline prevents contamination, electrode damage, and ensures the integrity of your experimental data.

Foundational Setup: Physical and Electrical Integrity
Proper setup is the bedrock of any successful electrochemical experiment. Errors in physical placement or electrical connection are a primary source of unreliable data.
Securing the Electrode
The electrode must be held firmly in the electrolytic cell or a dedicated holder. Ensure it is stable and not subject to mechanical vibrations, which can interfere with sensitive measurements.
Position the mesh at an appropriate distance from your reference and counter electrodes to ensure a uniform electric field and accurate potential readings.
Ensuring Proper Immersion
This is the single most critical rule: only the platinum mesh portion of the electrode should ever make contact with the electrolyte.
The upper parts of the electrode assembly, including where the platinum connects to the external rod or wire, often contain seals, adhesives, or solder points. Submerging these components can cause the seals to fail, leading to electrolyte seeping into the electrode body, contamination of your solution, and irreversible damage.
Making a Solid Electrical Connection
Use high-conductivity wires to connect the electrode to your power source or potentiostat. A poor or loose connection will introduce unwanted resistance and noise into your system, compromising your measurements.
Ensure the connection point is secure and corrosion-free.
Controlling the Experimental Environment
Your results are a direct function of the conditions you create. Strict control over the chemical and electrical environment is non-negotiable for achieving repeatability.
Selecting the Right Electrolyte
Choose an electrolyte that is chemically compatible with your reaction and, crucially, non-corrosive to platinum. While platinum is highly inert, aggressive media can still cause gradual degradation and contaminate your experiment.
Managing Current and Potential
Use a potentiostat or electrochemical workstation to precisely control the electrode's potential or the current flowing through it.
Avoid applying excessive current density (current per unit of surface area). Overly high currents can cause physical damage to the mesh, lead to undesirable side reactions, and reduce the lifespan of the electrode.
Regulating Temperature and Agitation
Most experiments are conducted at room temperature. If your procedure requires elevated temperatures, use a constant-temperature water bath and verify that your specific electrode model is rated for those conditions.
If stirring is required, maintain a constant and repeatable agitation speed, as this directly affects mass transport to the electrode surface and thus the measured current.
Understanding the Pitfalls and Trade-offs
Awareness of common failure modes is as important as knowing the correct procedure. These are the issues that most often compromise experiments.
The Risk of Over-Immersion
Submerging the electrode beyond the mesh is the most common and costly mistake. It not only risks immediate damage to the electrode's internal structure but also introduces subtle chemical contaminants from degrading seals, which can invalidate sensitive analytical work.
The Danger of Excessive Current Density
Pushing too much current through the electrode doesn't just speed up your experiment; it can fundamentally change it. It can damage the mesh, cause gas bubbles that block the active surface area ("blinding"), and initiate unintended electrochemical reactions that skew your results.
The Impact of Contamination
A contaminated electrode or electrolyte will produce unreliable data. Contamination can come from improper immersion, a corrosive electrolyte, or insufficient cleaning between experiments. Always monitor for signs of fouling or surface discoloration.
In-Situ Monitoring for Reliable Data
An experiment is a dynamic process. Pay close attention to the electrode's performance in real-time to catch problems as they happen.
Watching for Abnormalities
During the experiment, closely monitor the electrode's key performance indicators, such as its potential versus a reference electrode and the resulting current.
Sudden shifts, unexpected noise, or significant drift in these values can indicate a problem like electrode fouling, gas bubble formation, or a failing connection.
When to Stop and Inspect
If you observe any significant abnormalities, do not hesitate to pause the experiment. Carefully remove the electrode and inspect it for surface contamination, physical damage, or delamination of the platinum coating. It is far better to interrupt a run than to collect an entire set of invalid data.
A Checklist for Successful Operation
Your specific operational focus will depend on your experimental goal. Use these guidelines to prioritize your actions.
- If your primary focus is quantitative analysis: Ensure precise and repeatable control over immersion depth, temperature, and stirring speed to maintain consistent mass transport conditions.
- If your primary focus is long-term electrolysis: Carefully manage and limit the current density to prevent electrode degradation and maximize its operational lifespan.
- If your primary focus is preserving electrode lifespan: Adhere strictly to the rules of using non-corrosive electrolytes and never, under any circumstances, immersing the electrode beyond the active platinum mesh.
By treating the electrode with this methodical discipline, you ensure the collection of accurate data and protect the value of your instrumentation.
Summary Table:
| Key Operational Step | Critical Consideration |
|---|---|
| Securing & Immersion | Only the platinum mesh should contact the electrolyte to prevent damage and contamination. |
| Electrical Connection | Use high-conductivity wires for a secure, low-resistance connection to the potentiostat. |
| Electrolyte Selection | Must be non-corrosive to platinum to avoid degradation and contamination. |
| Current & Temperature Control | Avoid excessive current density and maintain stable temperature for repeatable data. |
| In-Situ Monitoring | Watch for abnormal potential/current shifts indicating fouling or damage. |
Achieve precise and reliable electrochemical data with confidence. Proper electrode operation is fundamental to your research success. KINTEK specializes in high-quality lab equipment and consumables, including robust electrochemical cells and accessories designed for accuracy and longevity. Let our experts help you optimize your setup. Contact our team today to discuss your specific laboratory needs and ensure your experiments are built on a foundation of quality.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Disc Electrode
People Also Ask
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements



















