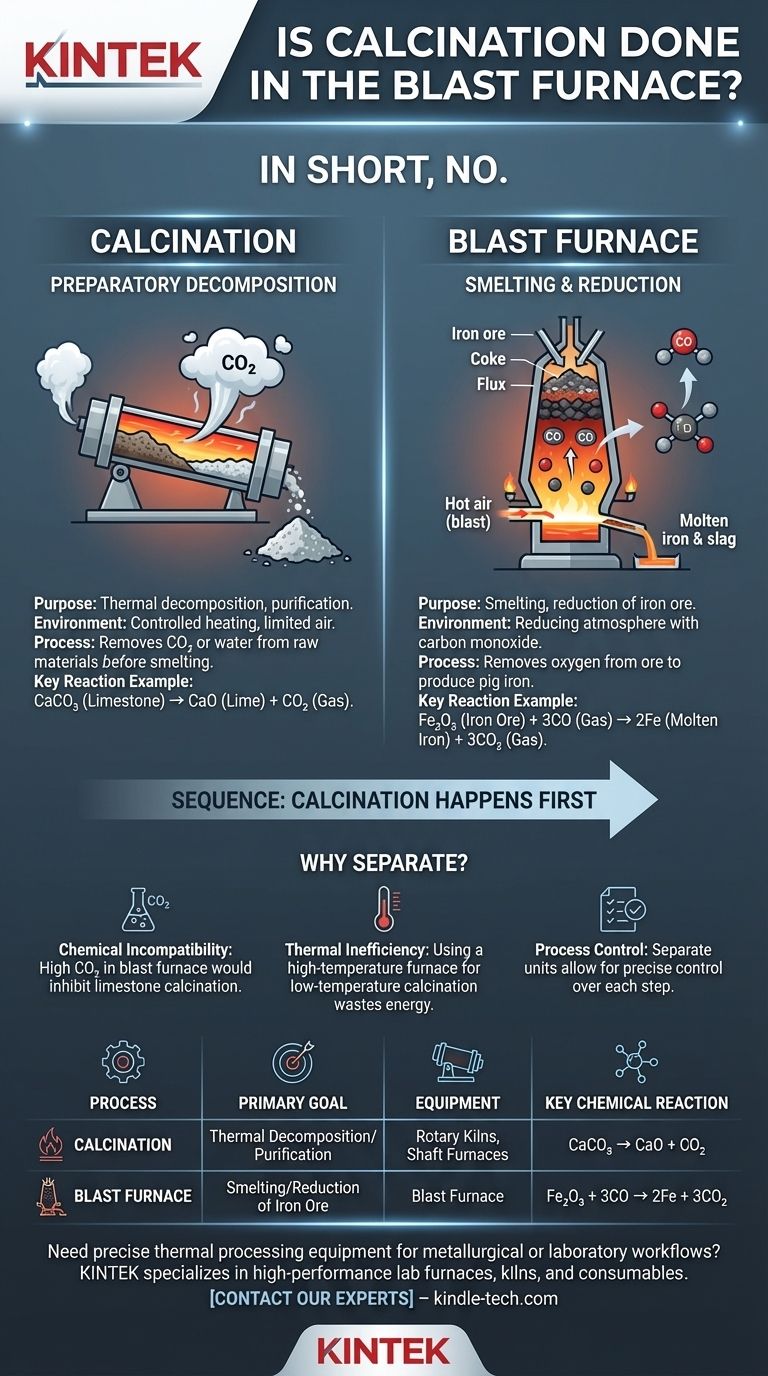
In short, no. Calcination is a preparatory thermal treatment that is not performed inside a blast furnace. The blast furnace is a highly specialized reactor designed for a different chemical purpose: the smelting and reduction of iron ore into molten pig iron. These two processes are distinct and occur in separate stages of metal production.
A blast furnace is engineered for reduction, using carbon monoxide to remove oxygen from iron ore. Calcination is a preceding step for decomposition, using heat to remove carbon dioxide or water from raw materials before they enter the blast furnace.

What is Calcination?
Calcination is a fundamental process in metallurgy and materials science that involves heating a solid material to a high temperature in the absence or limited supply of air. Its purpose is not to melt the material, but to cause thermal decomposition or drive off volatile substances.
The Goal: Purification and Decomposition
The primary goal of calcination is to purify the ore or prepare it for the next stage. A classic example relevant to iron production is the calcination of limestone (calcium carbonate, CaCO₃).
When heated, limestone decomposes into lime (calcium oxide, CaO) and carbon dioxide gas (CO₂). The resulting lime is a critical ingredient—a flux—that will later be added to the blast furnace.
The Environment: Controlled Heating
Calcination requires a specific environment, primarily focused on heat transfer. It is not about reacting the material with gases in the furnace atmosphere, but rather using heat to break chemical bonds within the material itself.
The Location: Rotary Kilns and Shaft Furnaces
Because of its specific requirements, calcination is carried out in dedicated equipment like rotary kilns or shaft furnaces. These vessels are designed to efficiently heat large volumes of material to precise temperatures, allowing volatile gases like CO₂ to escape before the material is charged into the blast furnace.
The Unique Role of the Blast Furnace
The blast furnace is the heart of an integrated steel mill, but its function is smelting, not calcination. It is essentially a giant, counter-current chemical reactor.
The Goal: Smelting and Reduction
The sole purpose of a blast furnace is to reduce iron oxides (the main component of iron ore) into liquid iron. This is a chemical transformation, not just a thermal one.
The Environment: A Reducing Atmosphere
A "blast" of hot air is injected into the bottom of the furnace, where it reacts with coke (a high-purity form of carbon) to produce intense heat and large quantities of carbon monoxide (CO) gas.
This CO gas is the primary reducing agent. As it rises through the furnace, it strips oxygen atoms from the descending iron ore, converting it to molten iron. This environment is the chemical opposite of what is required for roasting, which needs excess oxygen.
Understanding the Inefficiency: Why Processes are Kept Separate
Keeping calcination and smelting in separate units is not an accident; it is a deliberate engineering decision driven by chemical and thermal efficiency.
Chemical Incompatibility
A blast furnace operates with a high concentration of CO₂ and CO gases. Trying to calcinate limestone (CaCO₃ → CaO + CO₂) inside this environment would be highly inefficient. The high partial pressure of CO₂ in the furnace would suppress or even reverse the decomposition reaction, preventing the limestone from properly converting to lime.
Thermal Inefficiency
The blast furnace is an expensive, thermally optimized reactor designed for the extremely high temperatures needed to melt iron. Using it for a lower-temperature pre-treatment process like calcination would be a massive waste of energy and capacity. A rotary kiln can perform calcination far more economically.
Process Control
Separating the processes allows operators to have precise control over each step. The quality of the lime can be controlled in the kiln, and the reduction process can be optimized in the blast furnace without one interfering with the other. This modular approach leads to a more stable and efficient overall operation.
A Clear Sequence for Iron Production
To avoid confusion, it is best to view iron production as a logical sequence of distinct steps.
- If your primary focus is the overall process flow: View calcination as a preparatory step that happens before the charge (ore, coke, and flux) enters the blast furnace.
- If your primary focus is to distinguish between furnace types: Associate calcination with rotary kilns or shaft furnaces, and associate smelting/reduction with the blast furnace.
- If your primary focus is the core chemistry: Remember calcination uses heat to break down compounds, while the blast furnace uses a chemical agent (carbon monoxide) to reduce the ore to metal.
Understanding this deliberate separation of tasks is the key to mastering the logic of modern metallurgy.
Summary Table:
| Process | Primary Goal | Equipment Used | Key Chemical Reaction |
|---|---|---|---|
| Calcination | Thermal Decomposition / Purification | Rotary Kilns, Shaft Furnaces | e.g., CaCO₃ → CaO + CO₂ |
| Blast Furnace | Smelting / Reduction of Iron Ore | Blast Furnace | e.g., Fe₂O₃ + 3CO → 2Fe + 3CO₂ |
Need precise thermal processing equipment for your metallurgical or laboratory workflows? KINTEK specializes in high-performance lab furnaces, kilns, and consumables designed for processes like calcination. Our equipment ensures the efficiency and control your operations demand. Contact our experts today to find the perfect solution for your specific application!
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vertical Laboratory Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
People Also Ask
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- How does a high-temperature tube furnace facilitate the phase transformation of alumina products? Master Thermal Control
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.
- How do a quartz tube reactor and atmosphere furnace collaborate in Co@NC pyrolysis? Master Precision Synthesis
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?



















