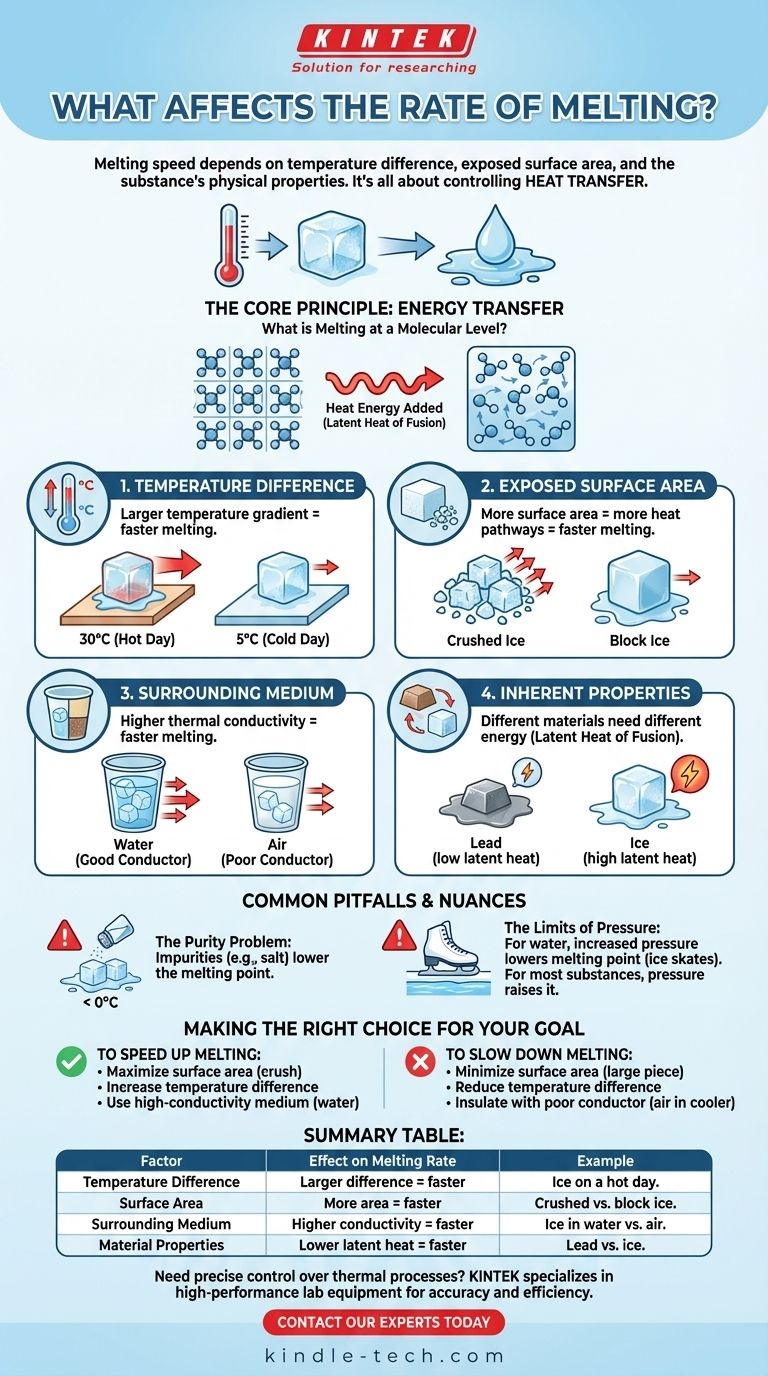
The rate at which a substance melts is not determined by a single factor, but by the interplay of several key variables. Primarily, melting speed depends on the temperature difference between the substance and its environment, the amount of exposed surface area, and the inherent physical properties of the substance itself.
At its core, controlling the rate of melting is about controlling the rate of heat transfer. The faster you can move energy into a solid to break its molecular bonds, the faster it will turn into a liquid.

The Core Principle: Energy Transfer
What is Melting at a Molecular Level?
In a solid, molecules are locked in a fixed, crystalline structure. They vibrate but cannot move freely.
Melting is the process of adding enough energy—usually in the form of heat—to break these bonds, allowing the molecules to move past one another as a liquid.
The Required Energy
This process requires a specific amount of energy known as the latent heat of fusion. Until this energy requirement is met, the substance will not fully melt.
Key Factors That Control Melting Speed
The Temperature Difference
The single most significant factor is the temperature gradient, or the difference in temperature between the solid and its surroundings.
A larger temperature difference creates a stronger "push" for heat energy to flow into the solid, accelerating the melting process. An ice cube will melt much faster on a 30°C (86°F) day than on a 5°C (41°F) day.
Exposed Surface Area
Heat can only transfer into an object through its surface. The more surface area is exposed, the more pathways are available for heat to enter.
This is why crushed ice melts significantly faster than a single, large block of ice of the same total mass. More surface area means a faster rate of energy absorption.
The Surrounding Medium
The substance surrounding the solid plays a critical role. Different materials transfer heat at different rates, a property known as thermal conductivity.
An ice cube will melt much faster in a cup of water than it will in air of the same temperature because water is a far better conductor of heat than air.
The Inherent Properties of the Substance
Not all solids are created equal. Different materials require different amounts of energy to melt, which is defined by their latent heat of fusion.
For example, melting one kilogram of lead requires far less energy than melting one kilogram of ice, even if both are at their respective melting points.
Common Pitfalls and Nuances
The Purity Problem
Impurities can drastically alter the melting process. Adding salt to ice, for example, disrupts the stable crystal structure of the water molecules.
This makes it easier for the bonds to break, effectively lowering the melting point and causing the ice to melt at temperatures below its normal 0°C (32°F).
The Limits of Pressure
For water, increasing pressure can lower the melting point. This is why an ice skate blade, which exerts high pressure on a small area, creates a thin layer of water that it glides upon.
However, for most other substances, increasing pressure actually raises the melting point, making it harder to melt. This effect is often minor compared to temperature and surface area.
Making the Right Choice for Your Goal
By understanding the principles of heat transfer, you can intentionally manipulate the melting process to fit your objective.
- If your primary focus is to speed up melting: Maximize surface area by crushing or breaking the solid, increase the ambient temperature, and use a surrounding medium with high thermal conductivity, like water.
- If your primary focus is to slow down melting: Use the largest single piece possible to minimize surface area, reduce the surrounding temperature, and insulate the solid with a poor heat conductor, like air in a cooler.
Ultimately, mastering these factors gives you direct control over this fundamental physical process.
Summary Table:
| Factor | Effect on Melting Rate | Example |
|---|---|---|
| Temperature Difference | Larger difference = faster melting | Ice melts faster on a hot day. |
| Surface Area | More area = faster melting | Crushed ice melts faster than a block. |
| Surrounding Medium | Higher conductivity = faster melting | Ice melts faster in water than in air. |
| Material Properties | Lower latent heat = faster melting | Lead melts faster than ice. |
Need precise control over melting, evaporation, or other thermal processes in your lab? KINTEK specializes in high-performance lab equipment, including furnaces, hot plates, and temperature control systems designed for accuracy and efficiency. Our solutions help you master heat transfer for consistent, reliable results. Contact our experts today to find the perfect equipment for your application!
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How much does a plastic waste pyrolysis plant cost? From $50K to $20M+
- What are zeolites advantages and disadvantages? Maximize Molecular Selectivity and Efficiency
- What is the purpose of a rotavap? Achieve Gentle, Efficient Solvent Removal for Your Lab
- What is the acceptable pressure drop across a filter? Master Your System's Health and Efficiency
- How does a controlled heating reaction system achieve morphology control for platinum nanoparticles?
- How does fast temperature recovery benefit ultra-low freezers? Protect Sample Integrity and Lab Efficiency
- What is the sputtering process for deposition? A Guide to Precision Thin Film Coating
- What is a pusher furnace? A Guide to Continuous High-Volume Thermal Processing



















