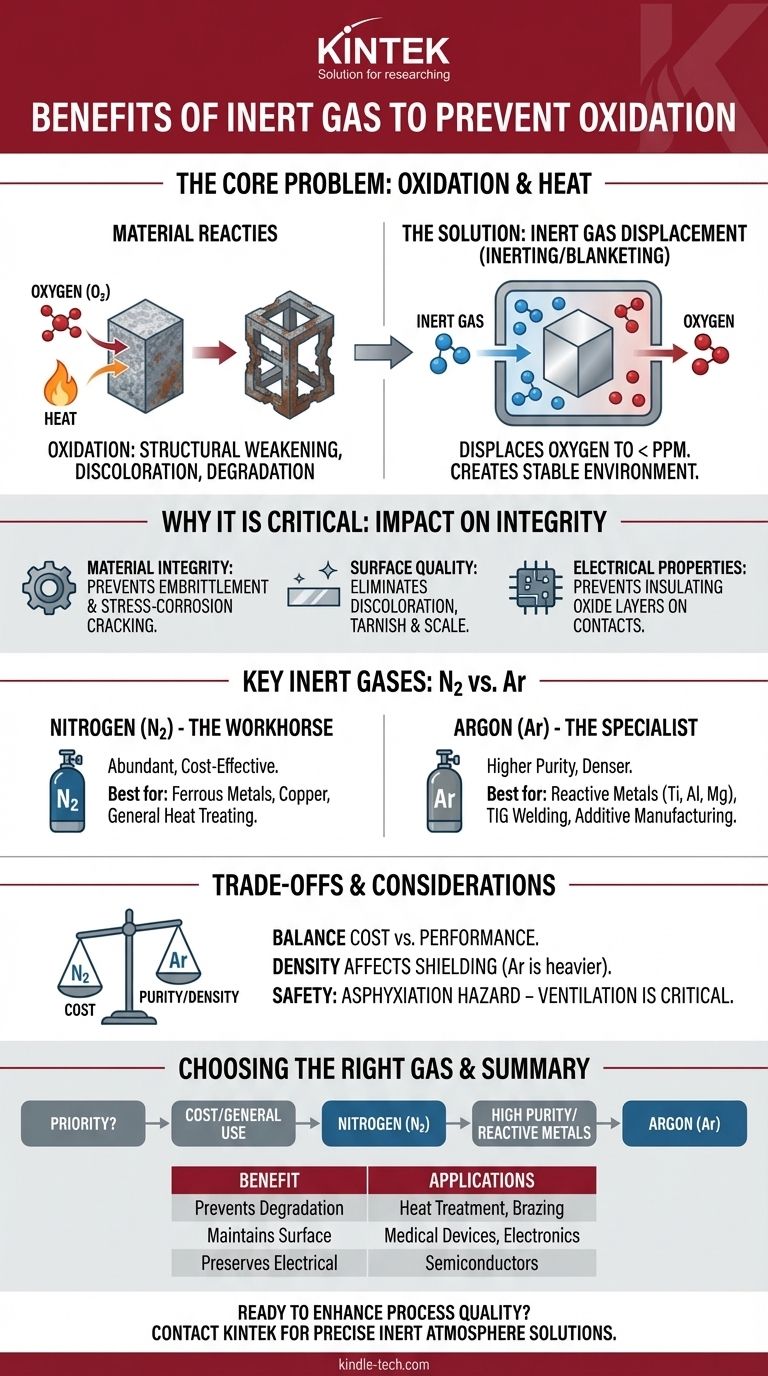
In any high-temperature industrial process, the primary benefit of using an inert gas is to create a chemically stable environment that protects materials from reacting with oxygen. This preventative measure, known as inerting or blanketing, is essential for stopping oxidation—a process that can cause irreversible damage such as structural weakening, discoloration, and the degradation of electrical properties.
Oxygen is a highly reactive element that will aggressively damage most materials, especially when heated. Using an inert gas is a foundational process control technique to displace this oxygen, thereby preserving the core structural, chemical, and aesthetic properties of the final product.

Why Preventing Oxidation is Critical
The decision to use an inert gas is not merely about preventing cosmetic rust. Oxidation is a fundamental chemical reaction that alters a material at a molecular level, with significant consequences for performance and quality.
Understanding the Core Problem: Oxidation
Oxidation is a chemical reaction where a substance loses electrons when it comes into contact with an oxidizing agent—the most common of which is the oxygen present in the ambient air. While this is famously seen as rust on iron, it affects nearly all materials, including polymers, ceramics, and other metals. Heat acts as a powerful catalyst, dramatically accelerating this destructive process.
The Impact on Material Integrity
For metals, oxidation can lead to embrittlement, reduced tensile strength, and a higher likelihood of stress-corrosion cracking. A component that has been unknowingly oxidized during a heat-treatment process like annealing or brazing may fail unexpectedly under load, even if it looks acceptable on the surface.
The Effect on Surface Quality
The most visible sign of oxidation is a change in surface appearance. This can range from simple discoloration and tarnishing to the formation of a thick, flaky layer of scale. In applications where aesthetics or precise surface finishes are paramount, such as in medical devices or consumer electronics, this is unacceptable.
The Change in Electrical Properties
In the manufacturing of electronics and semiconductors, oxidation is a critical failure point. An unwanted oxide layer on a conductive pad or contact can act as an insulator, preventing a proper electrical connection and causing device failure.
How Inert Gas Protection Works
The principle behind using an inert gas is simple but highly effective. By flooding a sealed environment, like an industrial oven or a welding area, with a non-reactive gas, you physically displace the oxygen.
The Principle of Displacement
An inert gas is one that does not readily participate in chemical reactions under a given set of conditions. By pumping a gas like nitrogen or argon into a chamber, you can reduce the oxygen concentration from its normal ~21% in air to mere parts per million (PPM), creating an "inert atmosphere." Without oxygen, the oxidation reaction cannot occur.
Nitrogen (N₂): The Industry Workhorse
Nitrogen is the most widely used inert gas due to its abundance (making up ~78% of Earth's atmosphere) and relatively low cost. It is effective for preventing oxidation in the heat treatment of many common metals like steel and copper.
Argon (Ar): The High-Purity Specialist
Argon is more inert than nitrogen and is chemically stable even at very high temperatures. It is the gas of choice for processing highly reactive metals like titanium, aluminum, and magnesium, or in advanced processes like TIG welding and metal 3D printing where even minimal reaction is unacceptable.
Understanding the Trade-offs
Choosing to use an inert gas is the first step. Selecting the right gas involves balancing performance, cost, and the specific needs of your material and process.
Cost vs. Purity
Nitrogen is significantly cheaper than Argon. For many applications, industrial-grade nitrogen provides sufficient protection. However, for extremely sensitive materials, the higher cost of high-purity Argon is a necessary investment to guarantee quality and prevent component failure.
Gas Density and Application
Argon is about 38% denser than air, while Nitrogen is slightly less dense. In applications like welding, Argon's density allows it to form a stable, heavy "blanket" over the work area. This physical property can sometimes influence which gas is more effective at shielding a specific part geometry.
The Myth of "Perfectly" Inert
While called "inert," these gases can react under extreme conditions. At very high temperatures, nitrogen can react with certain metals to form nitrides, which may be undesirable. Carbon Dioxide (CO₂), sometimes used as a cheap shielding gas, is not truly inert and can break down at high temperatures to form oxygen, actively contributing to oxidation where you're trying to prevent it.
Safety and Handling
All inert gases, with the exception of air itself, are asphyxiants. They displace oxygen not just from a process chamber but from the room they are in, creating a serious hazard for personnel if a leak occurs in an enclosed space. Proper ventilation, handling procedures, and oxygen monitoring are non-negotiable safety requirements.
Choosing the Right Gas for Your Application
The ideal gas depends entirely on your material, process temperature, budget, and final quality requirements.
- If your primary focus is cost-effective, general-purpose protection: Use Nitrogen (N₂) for most ferrous metals, copper, and general heat-treating applications.
- If your primary focus is processing highly reactive metals at high temperatures: Use Argon (Ar) to protect materials like titanium, aluminum, magnesium, and certain stainless steel alloys.
- If your primary focus is high-precision welding or additive manufacturing: Use high-purity Argon (Ar) to guarantee a completely stable and non-reactive environment for a perfect result.
- If your primary focus is balancing cost and performance: Consider a Nitrogen/Argon blend to get some benefits of Argon at a lower price point than using it pure.
By selecting the appropriate inert atmosphere, you are taking direct control over the final properties and quality of your material.
Summary Table:
| Benefit | Description | Key Applications |
|---|---|---|
| Prevents Material Degradation | Stops oxidation, avoiding embrittlement and loss of strength. | Heat treatment, annealing, brazing. |
| Maintains Surface Quality | Eliminates discoloration, tarnishing, and scale formation. | Medical devices, consumer electronics. |
| Preserves Electrical Properties | Prevents oxide layers that can cause electrical failure. | Electronics, semiconductor manufacturing. |
| Cost-Effective Protection | Nitrogen offers affordable oxidation prevention for many metals. | General industrial heat-treating. |
| High-Purity Processing | Argon provides superior inertness for reactive metals. | Titanium, aluminum processing, TIG welding. |
Ready to enhance your process quality with the right inert gas solution?
KINTEK specializes in providing high-performance lab equipment and consumables, including gas delivery systems designed for precise inert atmosphere control. Whether you need cost-effective nitrogen blanketing or high-purity argon for sensitive materials, our solutions help you prevent oxidation, improve product integrity, and reduce waste.
Contact us today to discuss your specific application and let our experts help you select the ideal equipment for your laboratory needs. Get in touch via our contact form to get started!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Why is high-purity argon needed for 12Kh18N10T steel processing? Protect Your Surface Integrity and Data Reliability
- What is the difference between oxidizing and reducing atmospheres? Key Insights for Your Applications
- Why is hydrogen annealing important? Achieve Bright, Stress-Free Metal Parts
- What are the potential dangers when working with inert gases? The Silent, Deadly Threat of Asphyxiation
- What is the role of an industrial electric heating oven in Fe-Cr-Mn-Mo-N-C steel production? Enhance SHS Stability
- What gases are used in a furnace? A Guide to Fuel vs. Process Atmospheres
- What is inert atmosphere used for? Prevent Oxidation and Ensure Process Safety
- How does the chemical reduction of silica during hydrogen sintering affect the furnace's refractory materials? Ensure Longevity with the Right Lining



















