Slow cooling fundamentally alters a material's internal structure, a process known as annealing. This procedure generally decreases hardness and tensile strength while significantly increasing ductility and toughness. By allowing the material's atoms sufficient time to rearrange into a stable, low-energy state, annealing relieves internal stresses and refines the grain structure, making the material more uniform and easier to work with.
The core principle of slow cooling is allowing a material's microstructure the time it needs to reach its most stable, equilibrium state. This results in a softer, more ductile, and less internally stressed material, trading raw strength for improved workability and toughness.

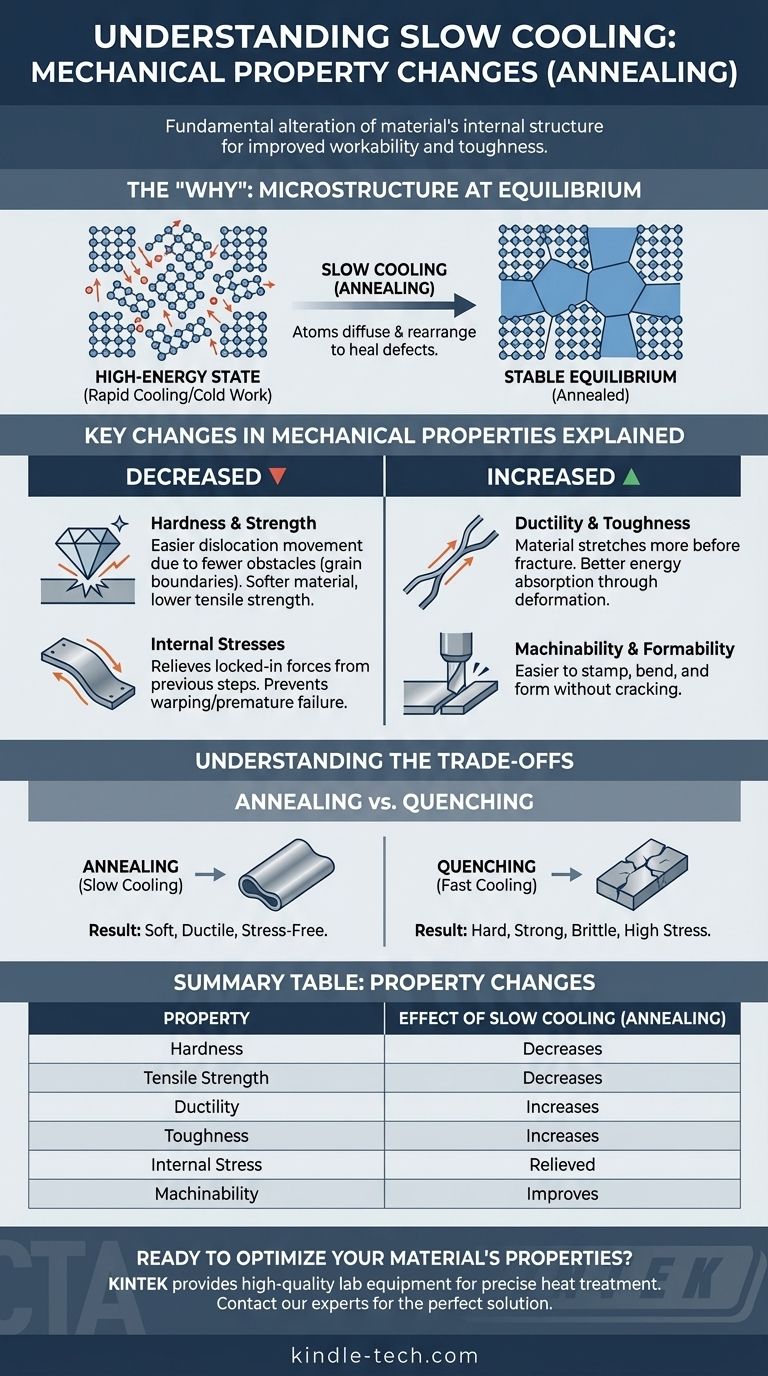
The "Why": Microstructure at Equilibrium
To understand the change in properties, you must first understand the change in the material's internal structure. Heat treatment is fundamentally about controlling this structure at a microscopic level.
The Goal of Slow Cooling: Reaching Stability
Processes like cold working (bending, rolling) or rapid cooling (quenching) trap a material in a high-energy, stressed state with numerous crystal lattice defects. Slow cooling, or annealing, is a controlled reversal that allows the material to relax into its most stable, ordered state.
Atomic Diffusion and Rearrangement
At elevated temperatures, atoms have enough energy to move, or diffuse, within the crystal lattice. By cooling slowly, you provide a long time window for this movement. Atoms migrate from strained positions to organize into well-ordered crystals, effectively "healing" microscopic defects.
The Resulting Coarse-Grained Structure
This slow, orderly process often results in the formation of larger, more uniform crystals, known as a coarse-grained structure. The size and orientation of these grains are a primary determinant of the material's final mechanical properties.
Key Changes in Mechanical Properties Explained
The shift towards a more stable, coarse-grained microstructure has direct and predictable consequences on a material's performance.
Decreased Hardness and Strength
Hardness and strength depend on how difficult it is for atomic planes to slip past one another. The large, uniform grains formed during slow cooling have fewer grain boundaries. Since grain boundaries act as obstacles to this slippage (dislocation movement), a coarse-grained structure offers less resistance, making the material softer and reducing its ultimate tensile strength.
Increased Ductility and Toughness
Ductility is the ability of a material to deform under tensile stress, like being drawn into a wire. With fewer internal defects and obstacles, atomic planes can slip more easily, allowing the material to stretch and deform significantly before fracturing. This ability to absorb energy through deformation also leads to an increase in toughness.
Relief of Internal Stresses
Internal stresses are locked-in forces from previous manufacturing steps like welding, casting, or rapid cooling. These stresses can cause warping or premature failure. Slow cooling provides the thermal energy and time for atoms to resettle into a relaxed configuration, effectively eliminating these internal stresses and creating a more stable component.
Understanding the Trade-offs: Annealing vs. Quenching
The effects of slow cooling are best understood when contrasted with its opposite: fast cooling, or quenching. The choice between them is one of the most fundamental trade-offs in materials engineering.
The Strength vs. Ductility Dilemma
This is the classic trade-off. Slow cooling (annealing) creates a soft and ductile material. Fast cooling (quenching) traps the microstructure in a chaotic, high-energy state (like martensite in steel), which is extremely hard and strong but very brittle.
Internal Stress as a Liability
Quenching induces massive internal stresses because different parts of the material cool and contract at different rates. This makes the part brittle and often necessitates a second heat treatment (tempering) to relieve some stress. Annealing is specifically designed to prevent this problem.
Machinability and Formability
A key practical benefit of slow cooling is vastly improved workability. The resulting soft and ductile material is much easier to machine, stamp, bend, or form without the risk of cracking. Hard, quenched materials are extremely difficult to work with.
How to Apply This to Your Goal
The choice between slow and fast cooling depends entirely on the desired final properties and the sequence of your manufacturing steps.
- If your primary focus is preparing for manufacturing: Choose slow cooling (annealing) to soften the material, making it easier to machine, stamp, or bend without cracking.
- If your primary focus is maximizing durability and toughness: Choose slow cooling to relieve internal stresses from prior processes like welding, preventing unexpected, brittle failures under load.
- If your primary focus is achieving maximum hardness and wear resistance: You would choose the opposite—fast cooling (quenching)—and likely follow it with a tempering process to manage the resulting brittleness.
Ultimately, understanding slow cooling is about controlling the material's internal structure to achieve a predictable and reliable engineering outcome.
Summary Table:
| Property Change | Effect of Slow Cooling (Annealing) |
|---|---|
| Hardness | Decreases |
| Tensile Strength | Decreases |
| Ductility | Increases |
| Toughness | Increases |
| Internal Stress | Relieved |
| Machinability | Improves |
Ready to optimize your material's properties with precise heat treatment?
At KINTEK, we specialize in providing high-quality lab equipment and consumables for all your laboratory needs. Whether you're annealing to improve machinability or quenching for maximum hardness, our reliable furnaces and expert support ensure you achieve consistent, predictable results.
Let us help you enhance your material's performance. Contact our experts today to discuss your specific application and discover the perfect solution for your lab.
Visual Guide
Related Products
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Vacuum Heat Treat Furnace and Levitation Induction Melting Furnace
- Vertical Laboratory Tube Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
People Also Ask
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality
- Why nitrogen is used in furnace? A Cost-Effective Shield for High-Temperature Processes
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- What are the functions of nitrogen (N2) in controlled furnace atmospheres? Achieve Superior Heat Treatment Results



















