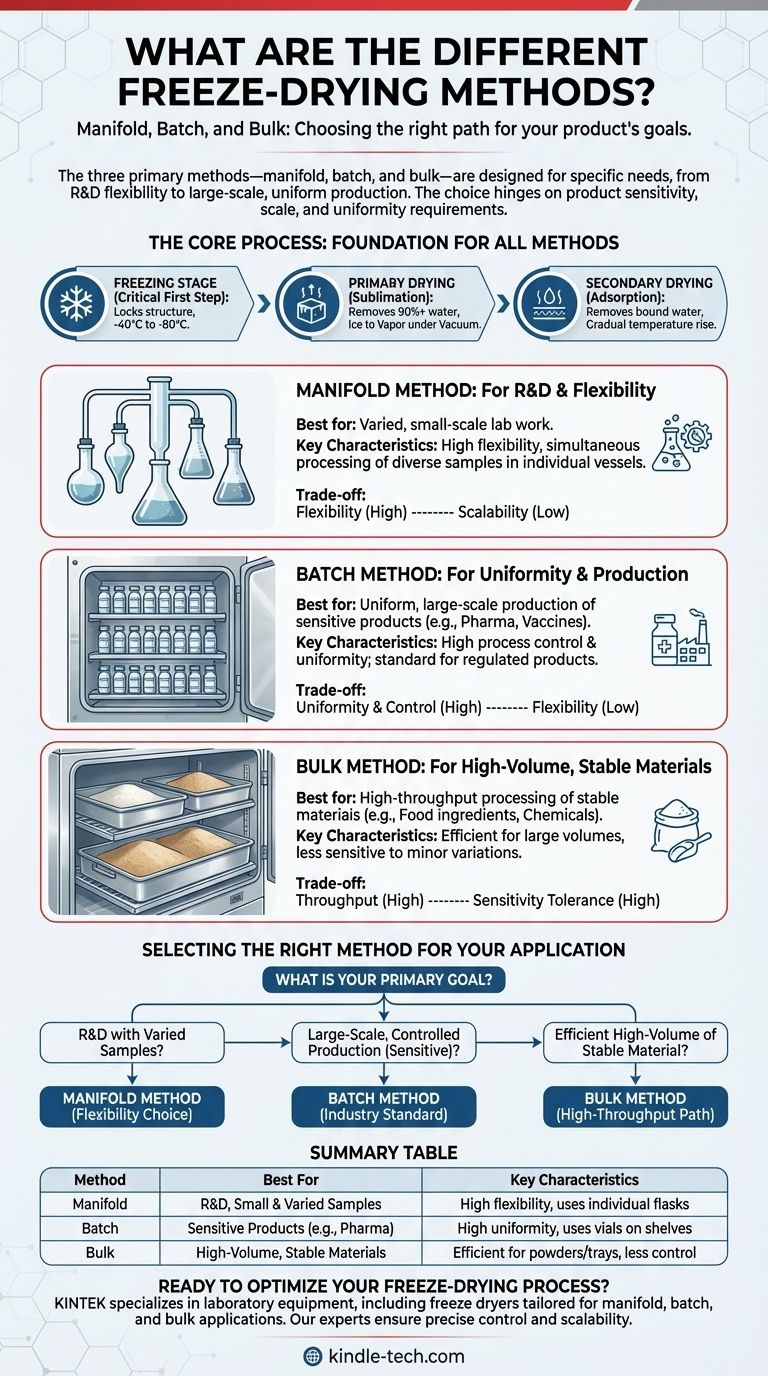
The three primary freeze-drying methods are manifold, batch, and bulk. Each method is designed for a specific purpose, varying in scale, flexibility, and the type of product being processed. The manifold method is best for varied, small-scale lab work, the batch method is the standard for uniform, large-scale production of sensitive products like pharmaceuticals, and the bulk method is used for high-volume processing of stable materials.
Choosing the right freeze-drying method is not about which is "best," but which is most appropriate for your specific goals. The decision hinges on three key factors: the sensitivity of your product, the required scale of production, and the need for process uniformity.

First, Understanding the Core Process
Before comparing the methods, it is crucial to understand the fundamental stages of freeze-drying, also known as lyophilization. Every method is simply a different way of managing this core process.
The Freezing Stage: The Critical First Step
The product is completely frozen, typically to a temperature between -40°C and -80°C. This step is essential because it locks water molecules in place as ice, ensuring the product's structure is maintained during drying.
The Primary Drying Stage (Sublimation)
Once frozen, a high-pressure vacuum is applied to the chamber. This allows the frozen water to turn directly from a solid (ice) into a gas (vapor) without passing through a liquid phase. This process, called sublimation, removes over 90% of the water content.
The Secondary Drying Stage (Adsorption)
After the free ice is gone, a small amount of "bound" water molecules remains attached to the product. The temperature is gradually raised while maintaining the vacuum, providing enough energy for these final water molecules to be released.
The Three Primary Freeze-Drying Methods
Each method applies the three-stage process differently to accommodate specific container types, product volumes, and control requirements.
The Manifold Method: For Flexibility and Research
This method involves connecting individual flasks or vessels directly to the ports of a manifold on the freeze dryer. It is highly flexible, allowing a researcher to dry different products in various container sizes simultaneously.
This approach is common in laboratory and research settings where sample variety is more important than uniform processing of a large single batch.
The Batch Method: For Uniformity and Production
In the batch method, products are loaded onto shelves inside the freeze-drying chamber. This is typically used for large numbers of identical containers, such as vials or ampules.
Every item in the batch experiences the exact same temperature and pressure conditions, ensuring high uniformity. This makes it the standard for producing sensitive biological and pharmaceutical products like vaccines, antibodies, and proteins.
The Bulk Method: For High-Volume, Stable Materials
The bulk method is used for processing materials in large, open trays. The product is loaded directly onto the trays, which are then placed on the shelves inside the dryer.
This method is reserved for stable products that are not highly sensitive to oxygen or minor variations in the process, such as certain food ingredients or less-sensitive chemical compounds.
Understanding the Trade-offs
Each method presents a distinct set of advantages and limitations. The optimal choice depends on balancing these factors against your project's requirements.
Flexibility vs. Scalability
The manifold method offers maximum flexibility for handling diverse samples but does not scale well for production. The batch and bulk methods are designed for scalability, processing large quantities of a single product efficiently.
Process Control and Uniformity
The batch method provides the highest degree of process control and uniformity, which is critical for regulated products like pharmaceuticals. Control over individual flasks in the manifold method is minimal, and the bulk method assumes the product can tolerate minor process variations.
Container and Product Type
Your container dictates your method. Vials and ampules are ideal for the batch method. Unique flasks and vessels require the manifold method. Powders or solids without individual containers are suited for the bulk method.
Selecting the Right Method for Your Application
Your final decision should be guided by your end goal.
- If your primary focus is research and development with varied samples: The flexibility of the manifold method is your ideal choice.
- If your primary focus is large-scale, controlled production of a sensitive product: The batch method provides the necessary uniformity and is the industry standard.
- If your primary focus is efficiently processing a large volume of a stable material: The bulk method offers the most straightforward path for high-throughput drying.
Ultimately, understanding these core methods empowers you to choose the optimal pathway to preserve your product's integrity and achieve your desired outcome.
Summary Table:
| Method | Best For | Key Characteristics |
|---|---|---|
| Manifold | R&D, Small & Varied Samples | High flexibility, uses individual flasks |
| Batch | Sensitive Products (e.g., Pharma) | High uniformity, uses vials on shelves |
| Bulk | High-Volume, Stable Materials | Efficient for powders/trays, less control |
Ready to Optimize Your Freeze-Drying Process?
Choosing the right method is critical for preserving your product's integrity and achieving your production goals. KINTEK specializes in providing laboratory equipment and consumables, including freeze dryers tailored for manifold, batch, and bulk applications.
Our experts can help you select the ideal equipment for your specific needs, whether you're in research, pharmaceuticals, or food processing. We ensure you get the precise control, scalability, and reliability your lab requires.
Contact us today via our contact form to discuss your project and discover how KINTEK can enhance your lyophilization workflow.
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- Why is a laboratory freeze-drying system essential for fermentation biomass? Preserve Sample Integrity for Analysis
- What is the function of a freeze dryer in the ice-templating process? Preserving Aligned Pore Scaffolds for LAGP
- Why is a freeze dryer preferred over thermal drying for Fe-ZTA cermets? Ensure Pure, Homogeneous Slurry Processing
- Why is a freeze dryer preferred for drying nickel nanoparticle precursors? Prevent Hard Agglomeration Now
- Why is a laboratory vacuum freeze dryer essential for plant extracts? Preserve Bioactivity & Structure



















