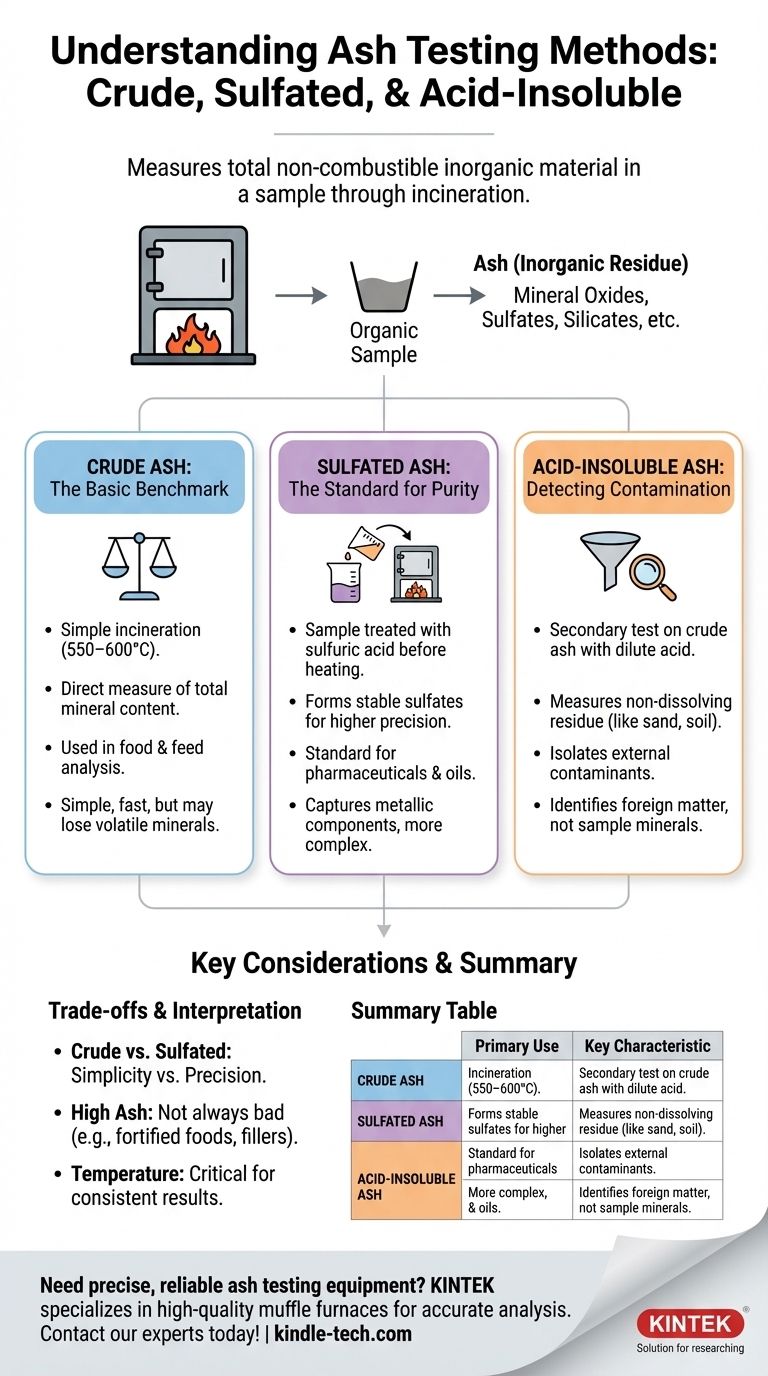
At its core, ash testing measures the total amount of non-combustible, inorganic material in a sample. The primary types are crude ash, which provides a general measure of total mineral content; sulfated ash, a more precise method used for pharmaceuticals and oils that stabilizes minerals before analysis; and acid-insoluble ash, a secondary test designed to detect external contaminants like sand or soil.
Choosing the right ash test is not about finding the "best" one, but about aligning the method with your specific analytical goal. The critical difference between the tests lies in what they are designed to include—or exclude—from the final measurement.

The Foundation: What Is Ash Testing?
Ash testing is a foundational technique in analytical chemistry, acting as a proxy for the total inorganic mineral content or the presence of inorganic impurities within an organic sample.
The Core Principle: Isolating Inorganic Residue
The process involves heating a sample at a very high temperature in a muffle furnace, a procedure known as incineration. This controlled burning removes all organic substances—like proteins, fats, and carbohydrates—by converting them to carbon dioxide and water.
The material left behind is a non-combustible residue called ash. This ash consists of mineral oxides, sulfates, phosphates, chlorides, and silicates, representing the inorganic portion of the original sample.
Why This Metric Is Critical
Ash content is a key quality and safety parameter across numerous industries.
- In food and animal feed, it indicates the nutritional mineral content but can also flag adulteration if the value is unexpectedly high.
- In pharmaceuticals, it is a critical purity test (often as sulfated ash) to quantify inorganic impurities in drug substances.
- In polymers and plastics, it measures the percentage of inorganic fillers like glass fiber or talc, which directly impacts the material's physical properties.
- In lubricating oils, it quantifies metal-containing additives that can form engine deposits.
A Breakdown of Key Ash Test Methods
While all methods involve incineration, subtle differences in procedure yield results tailored for different purposes.
Crude Ash: The Basic Benchmark
This is the most straightforward method. The sample is incinerated in a furnace at a specified temperature (typically 550–600°C) until all the organic matter is gone and the remaining ash achieves a constant weight.
This test provides a simple, direct measurement of the total mineral content. It is widely used in food analysis for nutritional labeling and basic quality control.
Sulfated Ash: The Standard for Purity and Additives
The sulfated ash method is more rigorous and is the standard for the pharmaceutical (USP <281>) and petroleum (ASTM D874) industries. Before the final high-temperature incineration, the sample is treated with sulfuric acid.
The acid converts metal salts and oxides into more thermally stable sulfates. This step prevents the loss of volatile metallic elements during heating, leading to a more accurate and repeatable measurement of the total inorganic matter, including metallic additives. The resulting ash weight is typically higher than that from a crude ash test.
Acid-Insoluble Ash: Detecting Contamination
This is a secondary test performed on the ash obtained from a crude ash analysis. The ash residue is mixed with a dilute acid, typically hydrochloric acid (HCl), and heated.
The portion of the ash that does not dissolve is filtered, dried, and weighed. This "acid-insoluble ash" primarily consists of silica from external sources like sand, soil, or dirt. It is a powerful tool for identifying external contamination that is not part of the sample's natural mineral profile.
Understanding the Trade-offs and Nuances
The data from an ash test is only valuable when its context and limitations are understood.
Crude Ash vs. Sulfated Ash: Simplicity vs. Precision
Crude ash is simpler, faster, and avoids the use of highly corrosive sulfuric acid. However, it can produce lower results if volatile minerals are lost during incineration.
Sulfated ash offers superior precision and repeatability, making it the required method for regulatory compliance in pharmaceuticals. It provides a truer measure of all metallic elements but is more complex and time-consuming.
Interpreting High vs. Low Ash Content
A "high" ash value is not inherently bad. For a mineral-fortified food or a glass-filled polymer, high ash is an expected part of the product's specification.
The key is whether the result is within the expected range. An unexpectedly high ash value often signals a problem, such as contamination (detected by acid-insoluble ash), adulteration, or a failure in the production process.
The Critical Role of Temperature
Different analytical standards (e.g., AOAC, USP, ASTM) specify different incineration temperatures. This is because certain minerals can decompose or volatilize at varying temperatures.
Using the incorrect temperature for your specified method will invalidate the results. Consistency in temperature is crucial for obtaining data that can be compared over time or between labs.
Selecting the Right Test for Your Goal
To choose the correct method, you must first define your analytical question.
- If your primary focus is general nutritional analysis or basic quality control: Crude ash is often sufficient for determining total mineral content in food and feed.
- If your primary focus is pharmaceutical purity or lubricant additive content: Sulfated ash is the required industry standard for its precision and ability to capture all metallic components.
- If your primary focus is detecting sand, soil, or siliceous contamination: Acid-insoluble ash is the definitive test to isolate and quantify external contaminants.
By understanding the purpose behind each method, you can transform ash analysis from a simple number into a powerful diagnostic tool for ensuring material quality and integrity.
Summary Table:
| Test Method | Primary Use | Key Characteristic |
|---|---|---|
| Crude Ash | General mineral content (food, feed) | Simple, direct measurement of total minerals |
| Sulfated Ash | Pharmaceutical purity, oil additives | Uses sulfuric acid for precise, stable results |
| Acid-Insoluble Ash | Contaminant detection (sand, soil) | Measures non-dissolving residue from crude ash |
Need precise, reliable ash testing equipment? KINTEK specializes in high-quality muffle furnaces and lab consumables designed for accurate crude, sulfated, and acid-insoluble ash analysis. Whether you work in food science, pharmaceuticals, polymers, or oils, our solutions ensure consistent, compliant results. Contact our experts today to find the perfect furnace for your lab's specific testing requirements!
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the principle of reactive sputtering? Create High-Performance Ceramic Coatings
- Can you provide a typical example of the calcination process? Discover the Limestone to Lime Transformation
- What are the different types of samples for XRF? Master Solid, Powder, and Liquid Preparation
- Why is temperature excursion alarming important in ultra-low freezers? Protect Your Valuable Samples from Catastrophic Loss
- Why is a high-precision constant temperature stirring reaction device necessary for functionalized BNNS grafting?
- How do baffled flasks and orbital shaker incubators facilitate yeast screening? Optimize Oxygen for Lipid Production
- What is the process used in semiconductor device fabrication? A Step-by-Step Guide to Microchip Manufacturing
- What is the rotary method of extraction? Harness High-Volume Thermal Processing for Your Materials



















