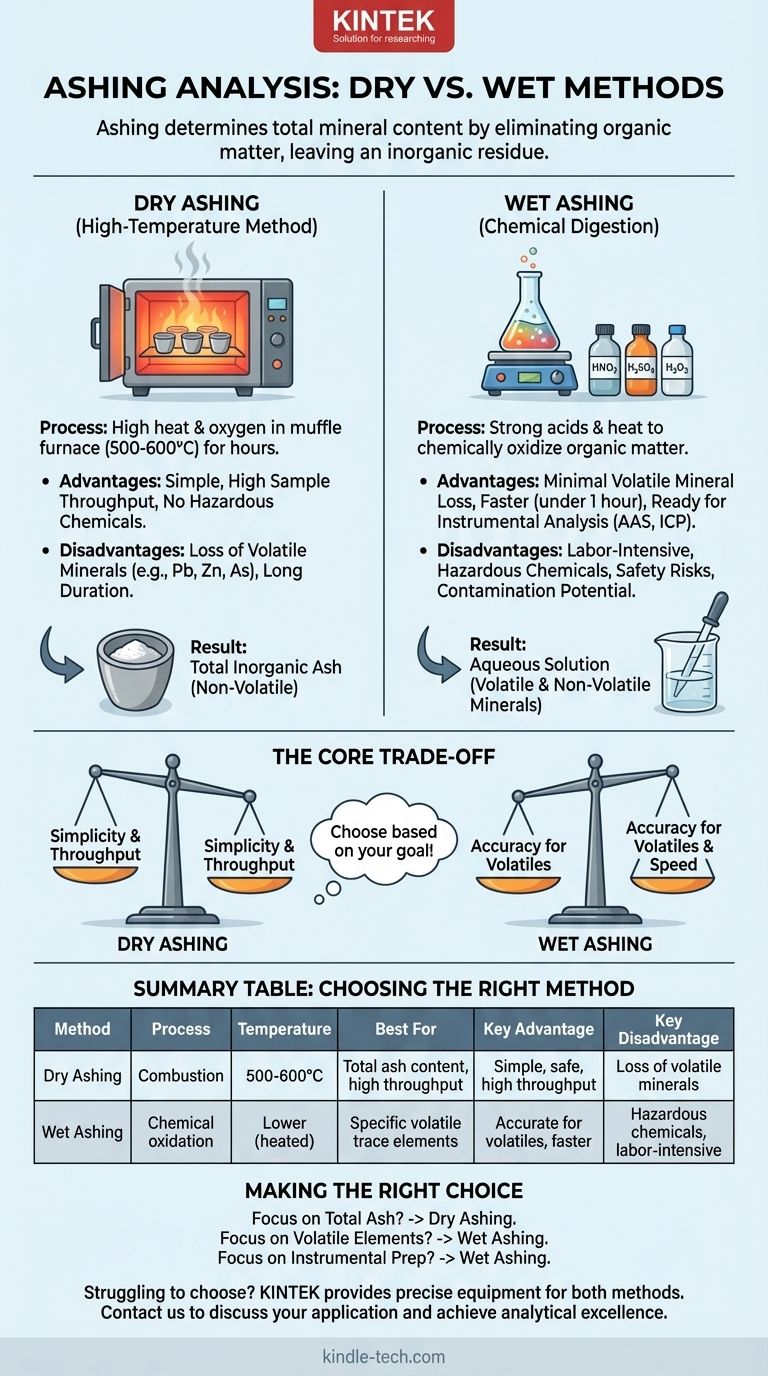
In analytical chemistry, the process of determining the total mineral content of a sample is known as ashing. The two primary methods for this analysis are dry ashing and wet ashing, also commonly referred to as wet digestion. Each technique uses a fundamentally different approach to eliminate the organic matter from a sample, leaving behind only the inorganic residue for measurement.
The core decision between dry and wet ashing hinges on a critical trade-off: balancing the need for high sample throughput and operational simplicity against the requirement to preserve volatile minerals for accurate elemental analysis.

What is Ashing and Why is it Performed?
Ashing is a crucial first step in many analytical procedures, designed to prepare a sample for further testing. It isolates the non-combustible components from the organic matrix that makes up most of the sample's weight.
The Fundamental Goal
The purpose of ashing is to measure the total amount of inorganic material in a sample. This inorganic residue, or "ash," consists of minerals like calcium, potassium, sodium, and magnesium, as well as trace elements.
Key Applications
This analysis is vital in various fields. In food science, it determines the nutritional mineral content for labeling. In environmental science, it helps measure heavy metal contamination in soil or water. In materials science, it serves as a quality control check for polymers and other products.
Dry Ashing: The High-Temperature Method
Dry ashing is the most common method for determining total ash content due to its simplicity. It leverages high heat and oxygen to systematically burn away all organic components.
The Process Explained
A sample is carefully weighed and placed in a ceramic or porcelain crucible. This crucible is then placed inside a muffle furnace, a specialized high-temperature oven. The temperature is raised to between 500 and 600°C, and the sample is held there for several hours until only a light gray or white ash remains.
Advantages of Dry Ashing
The primary advantage of this method is its simplicity. It requires minimal hands-on time and no hazardous chemical reagents. Furthermore, a muffle furnace can typically hold many crucibles, allowing for high sample throughput.
Disadvantages and Risks
The extremely high temperatures are the main drawback. Some important minerals, such as lead, zinc, and arsenic, are volatile and can be lost during the process, leading to inaccurate results for those specific elements. The long duration (often 4-18 hours) can also be a bottleneck.
Wet Ashing (Wet Digestion): The Chemical Approach
Wet ashing, or wet digestion, uses chemical oxidation to break down a sample. It is the preferred method when analyzing for specific trace minerals, especially those that are volatile.
The Process Explained
Instead of a furnace, wet ashing uses a combination of strong liquid oxidizing agents and applied heat. A sample is placed in a flask with acids like nitric acid, sulfuric acid, or hydrogen peroxide. The mixture is then gently heated to accelerate the decomposition of the organic material.
Advantages of Wet Ashing
Because it occurs at much lower temperatures than dry ashing, there is minimal to no loss of volatile minerals. The process is also significantly faster, often completed in under an hour. The resulting minerals are already in an aqueous solution, which is ideal for introduction into modern analytical instruments like Atomic Absorption Spectroscopy (AAS) or Inductively Coupled Plasma (ICP).
Disadvantages and Risks
This method is labor-intensive and requires constant supervision. It involves handling highly corrosive and hazardous acids, necessitating specialized safety equipment like a fume hood. There is also a higher risk of sample contamination from impurities present in the acids themselves.
Understanding the Trade-offs
Neither method is inherently superior; they are tools designed for different analytical goals. The right choice depends entirely on what you need to measure.
Speed vs. Throughput
Wet ashing is much faster per individual sample. However, dry ashing allows you to process a large batch of samples simultaneously with very little active labor, making it better for high-volume, non-critical analyses.
Accuracy vs. Safety
Wet ashing provides more accurate results for volatile trace elements. This accuracy comes at the cost of using dangerous reagents. Dry ashing is far safer but risks under-reporting any elements that can vaporize at high temperatures.
Target Analyte is the Deciding Factor
The most important question is: what are you trying to measure? If you only need the total ash percentage, dry ashing is simple and effective. If you need to know the precise amount of a specific volatile element like lead, wet ashing is the only reliable choice.
Making the Right Choice for Your Analysis
Your analytical objective should dictate your method. Consider the following guidelines when making your decision.
- If your primary focus is determining total ash content for quality control: Dry ashing offers the best combination of simplicity, safety, and high throughput.
- If your primary focus is analyzing for specific, volatile trace elements (like lead, mercury, or arsenic): Wet ashing is the required method to ensure accurate recovery and prevent mineral loss.
- If your primary focus is preparing a sample for subsequent instrumental analysis (like ICP-MS or AAS): Wet ashing is generally preferred as it is faster and leaves the analyte in a ready-to-analyze solution.
Choosing the appropriate ashing technique is the foundation for generating reliable and meaningful analytical data.
Summary Table:
| Method | Process | Temperature | Best For | Key Advantage | Key Disadvantage |
|---|---|---|---|---|---|
| Dry Ashing | High-temperature combustion in a muffle furnace | 500-600°C | Total ash content, high sample throughput | Simple, safe, high throughput | Loss of volatile minerals |
| Wet Ashing | Chemical oxidation with strong acids | Lower temperatures (heated) | Specific volatile trace elements (e.g., lead, arsenic) | Accurate for volatile elements, faster per sample | Hazardous chemicals, labor-intensive |
Struggling to choose the right ashing method for your lab's specific needs? KINTEK specializes in providing the precise lab equipment and consumables required for both dry and wet ashing analysis. Whether you need a reliable muffle furnace for high-throughput dry ashing or specialized glassware and safety equipment for wet digestion, our experts can help you select the ideal tools to ensure accurate and efficient results.
Contact us today via our [#ContactForm] to discuss your application and let KINTEK be your partner in achieving analytical excellence.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the structure of a muffle furnace? A Guide to Its Core Components and Design
- Why is an oven with air circulation required for polyester synthesis? Ensure Uniform Thermal Fields & Dense Networks
- What is an ashing furnace? A Key Tool for Precise Inorganic Material Analysis
- Is muffle furnace a vacuum? Choosing the Right High-Temperature Solution for Your Lab
- How is a high-temperature box resistance furnace utilized in the rejuvenation of P91 steel? Restore Material Integrity
- What is the purpose of the ash content test? A Guide to Material Quality Control
- How do you make biochar in a muffle furnace? A Step-by-Step Guide to Controlled Pyrolysis
- What function do high-temperature muffle or tube furnaces with inert atmosphere protection serve in alumina coating?



















