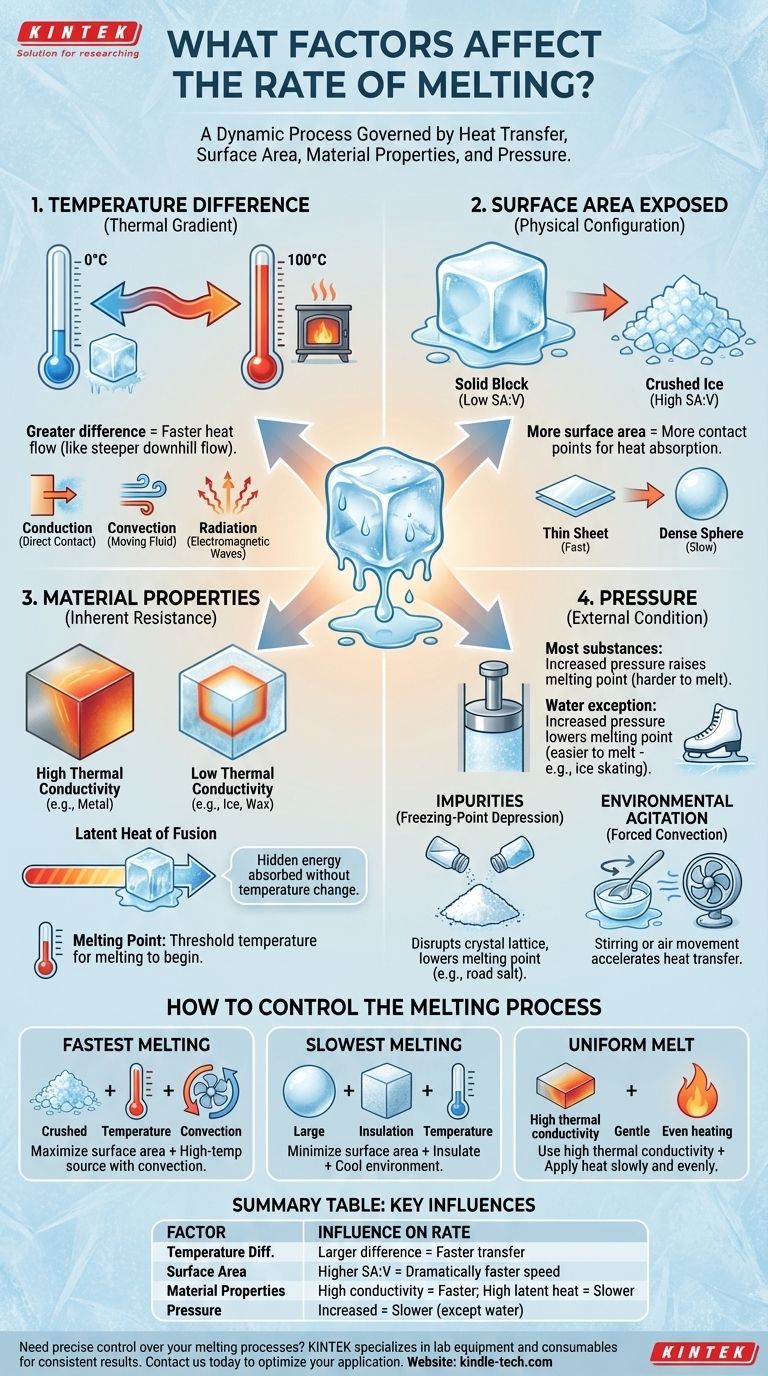
The rate at which a substance melts is not a fixed property but a dynamic process. It is governed by a combination of four primary factors: the temperature difference between the substance and its environment, the amount of surface area exposed, the inherent material properties of the substance itself (like thermal conductivity and latent heat), and the surrounding pressure.
Melting is fundamentally a process of heat transfer. To increase the melting rate, you must increase the speed at which a substance can absorb enough energy to break its internal bonds and transition from a solid to a liquid.

The Engine of Melting: Heat Transfer
The driving force behind any phase change from solid to liquid is the transfer of thermal energy. The speed of this transfer is the single most important element controlling the melting rate.
The Temperature Differential
The greater the difference between a substance's melting point and the temperature of its surroundings, the faster heat will flow into it. This is often called the thermal gradient.
Think of it like water flowing downhill. A steeper hill (a larger temperature difference) results in a faster flow of water (heat).
The Method of Heat Transfer
Heat can be transferred through three primary mechanisms, each impacting the melting rate differently.
Conduction involves direct contact, like a block of ice on a warm metal plate. Convection involves a moving fluid, like hot air from a hairdryer melting a plastic toy. Radiation involves electromagnetic waves, like the sun melting a snowman.
The Gateway for Heat: Physical Configuration
How a substance is shaped and arranged dramatically affects how quickly it can absorb the available heat from its environment.
Surface Area to Volume Ratio
Melting occurs at the surface of an object. By increasing the surface area, you create more points of contact for heat to enter the material.
This is why crushed ice melts dramatically faster than a solid block of ice of the same weight. The crushed ice has an enormous surface-area-to-volume ratio, allowing it to absorb ambient heat far more efficiently.
Overall Shape and Form
Even with the same mass and surface area, a substance's shape matters. A thin sheet will melt faster than a dense sphere.
In the sheet, no part of the material is very far from the surface where heat is being absorbed. In the sphere, heat must travel to the core, which takes more time.
A Substance's Inherent Resistance: Material Properties
Not all materials respond to heat in the same way. The unique physical properties of a substance dictate how it handles the energy required for melting.
Latent Heat of Fusion
This is the amount of "hidden" energy a substance must absorb to change from solid to liquid without any change in temperature.
A material with a high latent heat of fusion (like water) requires a substantial amount of energy to complete the phase change. It can absorb heat for a long time without its temperature rising above its melting point, making the process appear slow.
Thermal Conductivity
This property measures how efficiently a substance transfers heat from its surface to its interior.
Materials with high thermal conductivity, like most metals, distribute heat quickly throughout their structure, leading to a more uniform melt. Poor conductors, like ice or wax, melt slowly from the outside-in because the heat struggles to penetrate the core.
The Melting Point
While not a factor in the rate itself, a substance's melting point is the temperature threshold at which the melting process can begin. A substance with a lower melting point will naturally start melting in cooler environments.
Understanding the External Factors and Trade-offs
Beyond the core principles, external conditions can significantly alter the melting process, sometimes in counter-intuitive ways.
The Influence of Pressure
For most substances, increasing pressure forces molecules closer together, raising the melting point and making it harder to melt.
Water is a notable exception. Due to its unique crystalline structure, increasing pressure on ice actually lowers its melting point. This is the principle that allows an ice skate's blade to create a thin layer of water to glide on.
The Impact of Impurities
Adding impurities to a pure substance, like salt to ice, disrupts its uniform crystal lattice. This disruption makes it easier for the substance to melt, a phenomenon known as freezing-point depression.
This is why salt is used to de-ice roads. It doesn't generate heat, but it lowers the freezing point of water, causing existing ice to melt at temperatures below its normal 0°C (32°F).
Environmental Agitation
Stirring a melting substance or blowing air across its surface (forced convection) dramatically increases the melting rate. This action continuously replaces the cooler air or liquid at the substance's surface with warmer material, steepening the thermal gradient and accelerating heat transfer.
How to Control the Melting Process
Your strategy for controlling melting depends entirely on your desired outcome.
- If your primary focus is to melt something as quickly as possible: Maximize surface area by crushing or shaving it, and apply heat using a high-temperature medium with strong convection, like circulating hot air or liquid.
- If your primary focus is to slow down melting: Minimize surface area by using a large, compact shape (like a sphere), and insulate it from the warmer environment to reduce the rate of heat transfer.
- If your primary focus is to achieve a uniform, controlled melt: Use a substance with high thermal conductivity and apply heat slowly and evenly to all surfaces, allowing the energy to distribute throughout the object before it melts.
Understanding these principles transforms melting from a passive observation into a predictable and controllable physical process.
Summary Table:
| Factor | Key Influence on Melting Rate |
|---|---|
| Temperature Difference | A larger difference between the heat source and the material's melting point accelerates heat transfer. |
| Surface Area | A higher surface-area-to-volume ratio (e.g., crushed vs. solid) dramatically increases the melting speed. |
| Material Properties | High thermal conductivity speeds up melting; a high latent heat of fusion slows it down. |
| Pressure | For most materials, increased pressure raises the melting point, slowing the process. |
Need precise control over your melting processes?
Understanding the factors that affect melting rate is crucial for consistent results in research, material synthesis, and sample preparation. At KINTEK, we specialize in the lab equipment and consumables that give you this control—from high-temperature furnaces for uniform heating to specialized containers that manage surface area and heat transfer.
Let our experts help you select the right tools to optimize your specific application. Contact KINTEK today to discuss your laboratory needs and achieve reliable, efficient melting outcomes.
Visual Guide
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the difference between a furnace and an oven in a laboratory? Choose the Right Tool for Your Lab's Heat Needs
- What is a vertical muffle furnace used for? Achieve Superior Stability and Uniform Heating
- What is the annealing temperature of quartz? Achieve Optimal Thermal Stability for Your Components
- What is the use of furnace in laboratory? Unlock Material Transformation for Your Research
- What is the purpose of a laboratory furnace? Achieve Precise High-Temperature Processing



















