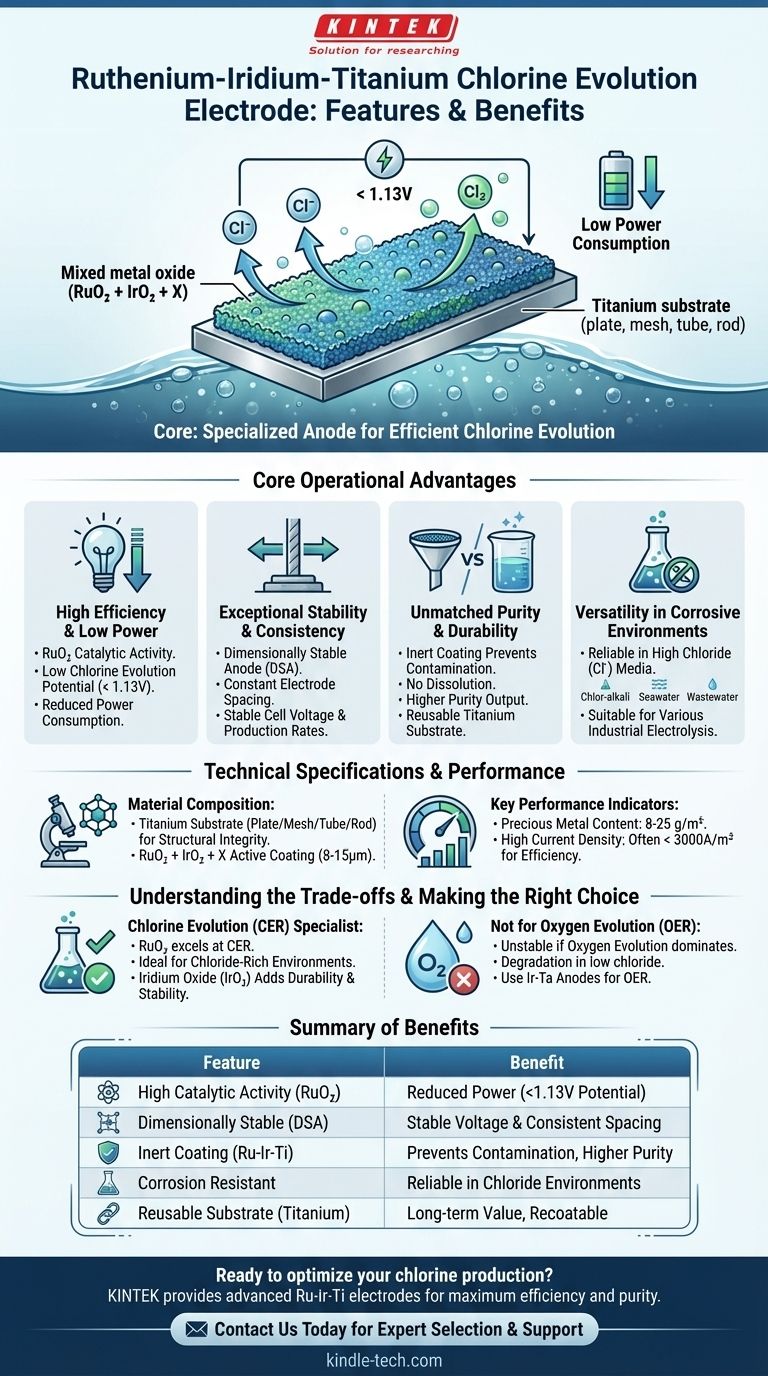
At its core, the Ruthenium-Iridium-Titanium (Ru-Ir-Ti) electrode is a highly specialized anode designed for exceptional performance in industrial chlorine evolution processes. Its key features include low power consumption, outstanding dimensional stability, a long operational lifespan, and the ability to prevent product contamination, making it a significant advancement over traditional graphite or lead anodes.
This electrode's primary value lies in its high catalytic activity specifically for the chlorine evolution reaction. This specialization delivers superior efficiency and stability in chloride-rich environments but also defines its operational limitations.

Core Operational Advantages
The features of the Ru-Ir-Ti electrode directly translate into tangible benefits for electrolytic processes, focusing on efficiency, consistency, and product purity.
High Efficiency and Low Power Consumption
The mixed metal oxide (MMO) coating containing Ruthenium Oxide (RuO₂) is highly catalytic for the chlorine evolution reaction.
This results in a low chlorine evolution potential (typically < 1.13V), which means less energy is required to drive the reaction. The direct consequence is lower working voltage and reduced overall power consumption.
Exceptional Stability and Consistency
These anodes are a type of Dimensionally Stable Anode (DSA). The titanium substrate and robust coating do not dissolve or change shape during electrolysis.
This stability maintains a constant electrode spacing, which is critical for operating at a stable cell voltage and achieving predictable, consistent production rates.
Unmatched Purity and Durability
The Ru-Ir-Ti electrode overcomes the primary drawback of older technologies like graphite and lead anodes, which gradually dissolve during operation.
This inertness prevents contamination of the electrolyte and final cathode products, ensuring a higher-purity output. Furthermore, the titanium substrate is reusable and can be recoated after the catalyst's lifespan is exhausted.
Versatility in Corrosive Environments
This electrode is engineered to perform reliably in highly corrosive electrolytic media, specifically those containing a high concentration of chloride ions (Cl⁻).
Its applications span the chlor-alkali industry, chlorate production, seawater electrolysis, and various forms of industrial wastewater treatment.
Technical Specifications and Performance
The performance of these electrodes is defined by a clear set of technical metrics that dictate their application and lifespan.
Material Composition
The electrode consists of a high-purity titanium substrate (available as a plate, mesh, tube, or rod) which provides structural integrity and corrosion resistance.
The active surface is a precisely applied coating of RuO₂ + IrO₂ + X, where "X" represents other proprietary stabilizing elements.
Key Performance Indicators
The typical coating thickness ranges from 8 to 15μm, with a precious metal content between 8 and 25 g/m².
These electrodes are designed to operate at a high current density, often below 3000A/m², which allows for high production efficiency.
Understanding the Trade-offs
No single electrode is perfect for every application. The high specialization of the Ru-Ir-Ti anode is both its greatest strength and its primary limitation.
The Specificity of Chlorine Evolution
The ruthenium oxide component is an exceptional catalyst for the chlorine evolution reaction (CER). It dramatically lowers the energy barrier for this specific chemical transformation.
However, RuO₂ is not stable when the oxygen evolution reaction (OER) becomes the dominant process. In environments lacking sufficient chloride ions, the coating will degrade rapidly.
The Role of Iridium
Iridium Oxide (IrO₂) is added to the coating primarily to enhance durability and stability. It provides better resistance to the small amount of oxygen evolution that inevitably occurs as a side reaction.
This synergy makes the coating robust for its intended purpose but does not make it a true oxygen evolution anode. For processes where oxygen is the desired product, an Iridium-Tantalum (Ir-Ta) based anode is the correct choice due to its superior stability in OER environments.
Making the Right Choice for Your Process
Your selection must be aligned with the specific chemical reaction you intend to facilitate.
- If your primary focus is efficient chlorine generation: The Ru-Ir-Ti electrode is the industry standard, offering an optimal balance of efficiency, stability, and lifespan.
- If you are upgrading from graphite or lead anodes: You will see immediate and significant improvements in energy consumption, operational consistency, and product purity.
- If your process involves significant oxygen evolution: You must use a different formulation, such as an Iridium-Tantalum (Ir-Ta) anode, to avoid rapid degradation of the electrode coating.
Ultimately, choosing this electrode means prioritizing world-class performance for a very specific and critical industrial reaction.
Summary Table:
| Feature | Benefit |
|---|---|
| High Catalytic Activity (RuO₂) | Low chlorine evolution potential (<1.13V) for reduced power consumption |
| Dimensionally Stable Anode (DSA) | Consistent electrode spacing and stable cell voltage |
| Inert Coating (Ru-Ir-Ti) | Prevents product contamination, unlike dissolving graphite/lead anodes |
| Corrosion Resistant | Reliable performance in chloride-rich environments (e.g., chlor-alkali, seawater) |
| Reusable Substrate | Titanium base can be recoated after catalyst lifespan, enhancing long-term value |
Ready to optimize your chlorine production with superior electrode technology?
KINTEK specializes in high-performance lab equipment and consumables, including advanced electrodes for industrial electrolysis. Our Ru-Ir-Ti anodes are engineered to deliver maximum efficiency, purity, and durability for your specific laboratory or production needs.
Contact us today to discuss how our solutions can enhance your process efficiency and product quality. Let our experts help you select the perfect electrode for your application.
Get in touch with our team now!
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Anion Exchange Membrane for Laboratory Use
- Custom PTFE Wafer Holders for Lab and Semiconductor Processing
People Also Ask
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data
- What is a common use for a platinum sheet electrode? As a Reliable Counter Electrode in Electrochemical Cells
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- How should a platinum wire/rod electrode be installed? Ensure Accurate Electrochemical Measurements
- What are the functions of platinum sheet and Ag/AgCl electrodes in corrosion testing? Master Electrochemical Precision



















