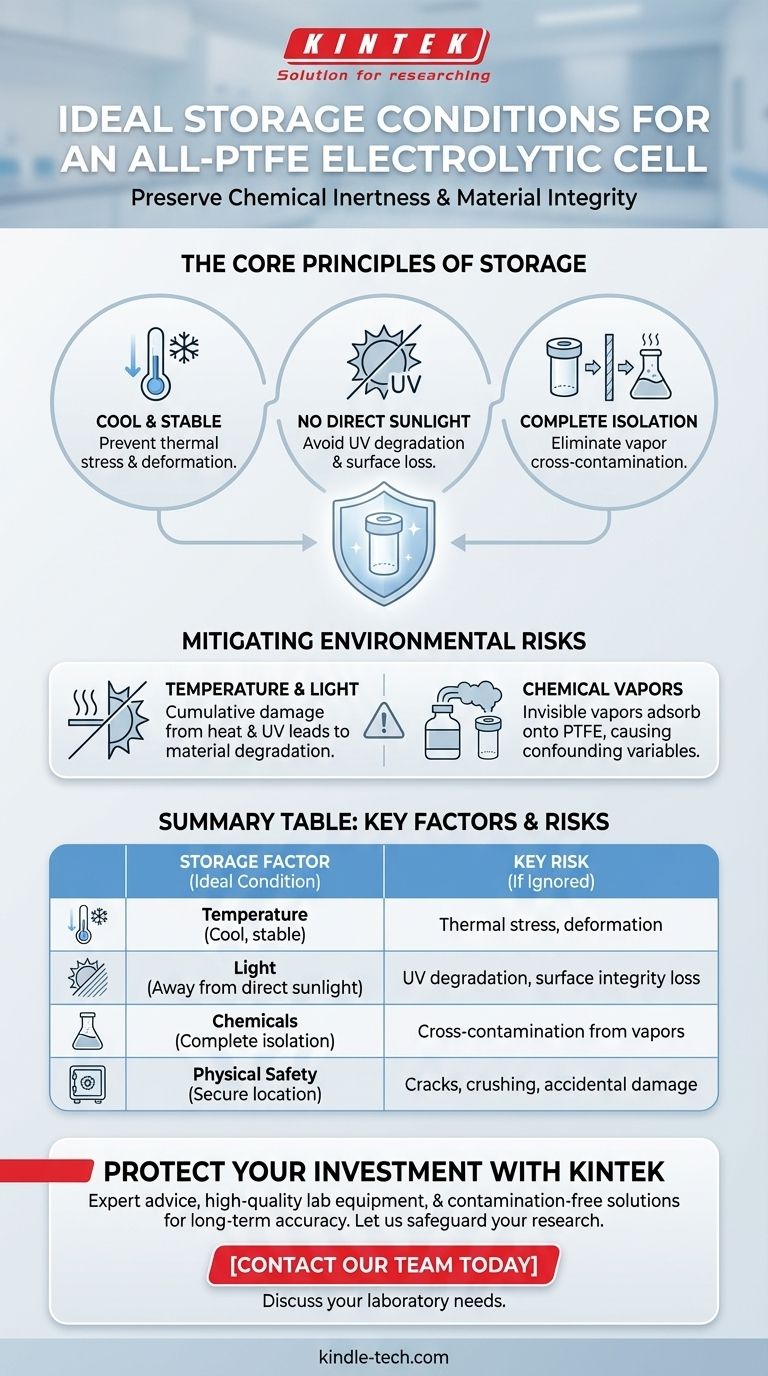
To properly store an all-PTFE electrolytic cell, you must keep it in a cool, dry, and ventilated environment. It is critical to store it away from direct sunlight and high temperatures, and completely isolated from other chemical substances to prevent reactions or contamination.
The goal of proper storage extends beyond simply protecting the cell from physical damage. It is fundamentally about preserving the unique chemical inertness and non-contaminating surface of the PTFE, which is the primary reason for using this material in sensitive electrochemical applications.

The Principles of PTFE Cell Storage
An all-PTFE electrolytic cell is a precision instrument. Its value lies in its extreme chemical resistance and the purity it brings to an experiment. Storage protocols are designed to protect these two critical attributes: material integrity and chemical purity.
Mitigating Environmental Degradation
While PTFE is exceptionally robust, it is not indestructible. Long-term exposure to certain environmental factors can slowly degrade the material.
Temperature is a key factor. Storing the cell in a cool, stable environment prevents any risk of thermal stress or deformation that could occur near heat sources.
Sunlight, specifically its ultraviolet (UV) component, can degrade polymers over time. Prolonged exposure can lead to discoloration and a potential loss of surface integrity, compromising the cell's non-contaminating properties.
Preventing Physical Damage
This is the most straightforward aspect of storage. The cell should be stored in a dedicated, secure location where it will not be dropped, crushed, or have heavy items placed on it.
A designated cabinet or a sturdy, labeled box is an ideal solution. This simple step prevents the most common forms of accidental damage.
Ensuring Chemical Purity
This is the most critical principle for any researcher. The primary advantage of a PTFE cell is its inertness, which prevents the cell itself from contaminating your experiment.
Improper storage can completely negate this benefit. Storing the cell near other chemicals, even if they are in sealed containers, risks cross-contamination from vapors that can adsorb onto the PTFE surface.
Common Pitfalls to Avoid
Understanding what can go wrong is as important as knowing the correct procedure. In our experience, two primary mistakes lead to damaged cells or compromised experiments.
The Risk of Cross-Contamination
This is the most insidious risk. A researcher might assume that since the cell is clean and dry, it can be stored on a general laboratory shelf.
However, volatile organic compounds or acid vapors from nearby containers can easily adsorb onto the PTFE. This contamination may be invisible but can leach into your next experiment, creating confounding variables and invalidating your results. Isolating the cell is non-negotiable.
The Danger of Thermal and UV Damage
Leaving a cell on a windowsill or near a heating vent is a common mistake. While the damage from sunlight or moderate heat is not immediate, it is cumulative.
Over months or years, this exposure will degrade the material. For an instrument intended to last, this slow degradation represents a significant loss of investment and a future source of experimental error.
Making the Right Choice for Storage
Your storage strategy should align directly with your primary goal, whether that is ensuring immediate experimental success or long-term asset preservation.
- If your primary focus is experimental accuracy: The most critical action is to store the cell in a location physically separate from all other chemical sources to guarantee its chemical purity.
- If your primary focus is long-term asset preservation: Protecting the cell from direct sunlight and temperature extremes is essential to prevent the slow degradation of the PTFE material over time.
Ultimately, proper storage is a simple discipline that protects both your equipment and the integrity of your results.
Summary Table:
| Storage Factor | Ideal Condition | Key Risk if Ignored |
|---|---|---|
| Temperature | Cool, stable environment | Thermal stress, material deformation |
| Light | Away from direct sunlight | UV degradation, loss of surface integrity |
| Chemicals | Complete isolation | Cross-contamination from vapors |
| Physical Safety | Secure, dedicated location | Cracks, crushing, accidental damage |
Protect your investment and ensure experimental purity with KINTEK.
Proper storage is just one part of maximizing the performance and lifespan of your lab equipment. At KINTEK, we specialize in high-quality lab equipment and consumables, including precision instruments like PTFE electrolytic cells. Our experts understand the critical need for chemical inertness and material integrity in sensitive applications.
Let us help you safeguard your research:
- Get expert advice on storage solutions and best practices.
- Explore our range of reliable, contamination-free lab equipment.
- Ensure long-term accuracy for your most demanding electrochemical experiments.
Contact our team today to discuss your laboratory needs and discover how KINTEK can support your success.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tube Racks
- Custom PTFE Teflon Parts Manufacturer for Conductive Glass Substrate Cleaning Rack
- Custom PTFE Teflon Parts Manufacturer for Gaskets and More
People Also Ask
- Why is a quartz electrolytic cell used for acrylic acid wastewater? Ensure Chemical Stability & Data Integrity
- What are the standard opening specifications for sealed and unsealed all-quartz electrolytic cells? Optimize Your Electrochemistry Setup
- What are the operational procedures and safety precautions during an experiment using an all-quartz electrolytic cell? Ensure Safety and Accuracy in Your Lab
- What precautions should be taken when handling and using an all-quartz electrolytic cell? Ensure Safe, Accurate, and Durable Performance
- What are the primary applications of the all-quartz electrolytic cell? Essential for High-Purity & Optical Analysis



















