At its core, a glassy carbon electrode is a high-performance tool for electrochemistry, valued for its unique combination of properties. It possesses high electrical conductivity, exceptional chemical inertness across a wide potential window, and a surface that is easily modified, making it a standard choice for electrochemical analysis, sensor development, and biomedical research.
Glassy carbon's value stems from its paradoxical nature: it combines the disordered, inert structure of glass with the excellent electrical conductivity of a metal-like carbon. This makes it an exceptionally stable and versatile platform for studying chemical reactions with electricity.

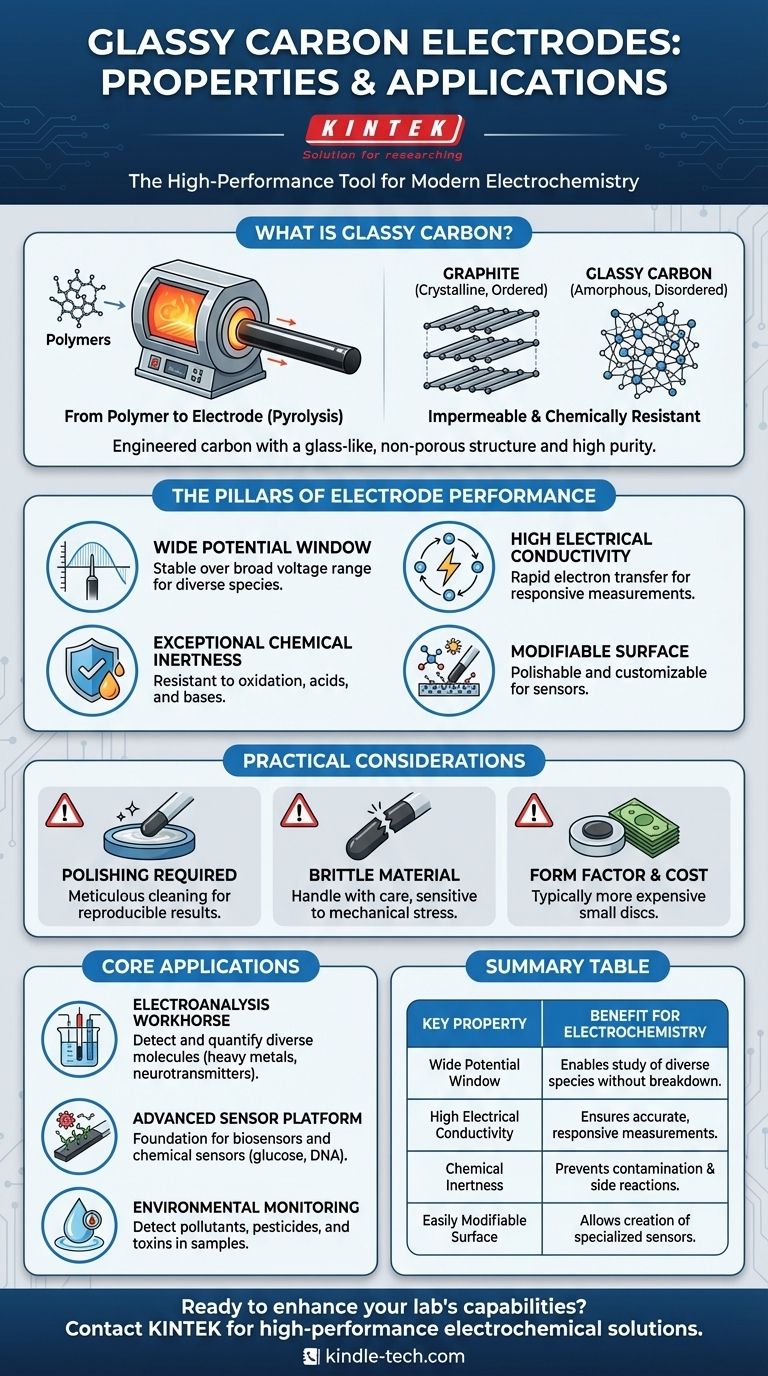
Deconstructing the Material: What is Glassy Carbon?
From Polymer to Electrode
Glassy carbon is not a naturally occurring material. It is an engineered substance created through the high-temperature pyrolysis (thermal decomposition in an inert atmosphere) of specific polymers, such as phenolic resin.
This controlled process burns off non-carbon elements, resulting in a product of very high purity and density. The final material has a uniform, non-porous microstructure.
A Disordered, 'Glass-Like' Structure
Unlike graphite, which has an ordered, crystalline layered structure, glassy carbon is amorphous. Its carbon atoms are arranged in a disordered, tangled network, similar to the atomic structure of common glass.
This lack of crystalline grain boundaries is a key reason for its exceptional properties, including its impermeability to gases and its high chemical resistance.
The Pillars of Electrode Performance
The utility of a glassy carbon electrode in electrochemistry is built on four fundamental properties that work in concert.
Wide Potential Window
A wide potential window means the electrode itself remains stable and non-reactive over a broad range of applied voltages. This is critical because it allows researchers to study a wide variety of chemical species without interference or breakdown of the electrode material itself.
High Electrical Conductivity
Efficient electrochemistry requires rapid electron transfer between the electrode and the analyte in solution. Glassy carbon offers excellent electrical conductivity, ensuring that the measurements are accurate and responsive to the chemical changes occurring at the surface.
Exceptional Chemical Inertness
The material exhibits remarkable resistance to oxidation and attack from strong acids and bases. This inertness ensures that the electrode does not contaminate the experiment or produce unwanted side reactions, leading to cleaner, more reliable data.
A Modifiable Surface
The surface of a glassy carbon electrode can be easily polished to a mirror finish, providing a reproducible starting point for experiments. More importantly, this surface can be chemically or electrochemically modified to attach specific molecules, nanoparticles, or enzymes, turning the simple electrode into a highly specialized sensor.
Understanding the Trade-offs and Practical Use
While highly effective, glassy carbon is not without its practical considerations. Understanding these ensures proper handling and optimal experimental results.
The Importance of Polishing
The electrochemical response is highly sensitive to the state of the electrode surface. Before each use, the electrode must be meticulously polished with an alumina slurry to remove any adsorbed species from previous experiments and expose a fresh, clean surface. Failure to do so is a common source of irreproducible results.
Brittleness and Mechanical Stress
Although glassy carbon has a hardness approaching that of a diamond, it is also brittle, much like the glass it is named after. Dropping the electrode or applying significant mechanical stress can cause it to fracture, requiring a costly replacement.
Form Factor and Cost
Glassy carbon electrodes are most commonly produced as small discs (2-5 mm diameter) embedded in an insulating sheath like PEEK or Teflon. While they offer superior performance, they are generally more expensive than other carbon-based electrodes, such as those made from carbon paste or pyrolytic graphite.
Where Glassy Carbon Excels: Core Applications
The unique properties of glassy carbon make it the go-to working electrode in numerous scientific and industrial fields.
The Workhorse of Electroanalysis
In a standard three-electrode setup, the glassy carbon electrode serves as the working electrode—the site where the reaction of interest occurs. It is used to detect and quantify a vast range of molecules, from heavy metals in water to neurotransmitters in biological samples.
Platforms for Advanced Sensors
Its easily modifiable surface makes it an ideal foundation for creating biosensors and chemical sensors. By attaching enzymes, antibodies, or specific polymers, researchers can design electrodes that respond selectively to a single target analyte, such as glucose or a specific DNA sequence.
Environmental Monitoring
The sensitivity and stability of glassy carbon electrodes are leveraged for environmental science. They are used to detect low concentrations of pollutants, pesticides, and toxic heavy metals in water, soil, and air samples.
Making the Right Choice for Your Goal
- If your primary focus is sensitive electroanalysis: Its wide potential window and low background current make it the ideal choice for detecting trace analytes with high signal-to-noise.
- If your primary focus is developing novel sensors: Its easily functionalized surface provides a stable and reliable platform for building complex sensing architectures.
- If you need a robust, general-purpose working electrode: Glassy carbon's combination of inertness, conductivity, and reproducibility offers superior performance for a wide range of electrochemical experiments.
Ultimately, the engineered properties of glassy carbon make it an indispensable and reliable tool for advancing modern electrochemistry.
Summary Table:
| Key Property | Benefit for Electrochemistry |
|---|---|
| Wide Potential Window | Enables study of diverse chemical species without electrode breakdown |
| High Electrical Conductivity | Ensures accurate, responsive measurements |
| Chemical Inertness | Prevents contamination and unwanted side reactions |
| Easily Modifiable Surface | Allows creation of specialized sensors and biosensors |
Ready to enhance your lab's electrochemical capabilities?
KINTEK specializes in high-performance lab equipment and consumables, including glassy carbon electrodes and related electrochemical systems. Our products are designed to deliver the reliability and precision your research demands, whether you're conducting sensitive electroanalysis, developing novel biosensors, or performing environmental monitoring.
Let us help you achieve cleaner data and more reproducible results. Contact our experts today to discuss your specific laboratory needs and discover how our solutions can power your next breakthrough.
Visual Guide
Related Products
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Electrode Polishing Material for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What applications is carbon felt suitable for? Ideal for High-Performance Electrochemical Systems
- What are the common applications for carbon cloth? Unlock Its Potential in Energy & Electrochemical Systems
- How should carbon cloth used for high-temperature electrolysis be handled after operation? Prevent Irreversible Oxidative Damage
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- Why are high surface area materials preferred for BES anodes? Maximize Microbial Power and Efficiency



















